Guidelines on the Management of Patients with Vestibular Schwannoma
7. The Role of Radiosurgery and Radiation Therapy in the Management of Patients with Vestibular Schwannomas
download pdf Neurosurgery, 2017
Sponsored by: Congress of Neurological Surgeons (CNS) and the AANS/CNS Tumor Section
Endorsed by: Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)
Authors:
Isabelle M. Germano, MD1, Jason Sheehan, MD2, PhD, Johnathan Parish, MD3, Tyler Atkins, MD3, Anthony Asher, MD4, Constantinos G. Hadjipanayis, MD, PhD1, Stuart H. Burri, MD5, Sheryl Green, MBBCh6, Jeffrey J. Olson, MD7
1 Department of Neurological Surgery, Icahn School of Medicine at Mount Sinai, New York, New York, USA
2 Department of Neurological Surgery, University of Virginia, Charlottesville, Virginia, USA
3 Carolinas Medical Center, Charlotte, North Carolina, USA
4 Carolina Neurosurgery & Spine Associates, Charlotte, North Carolina, USA
5 Department of Radiation Oncology, Levine Cancer Institute, Charlotte, North Carolina, USA
6 Department of Radiation Oncology, Icahn School of Medicine at Mount Sinai, New York, New York, USA
7 Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
Correspondence:
Isabelle M. Germano, MD
Department of Neurological Surgery Box 1136
Icahn School of Medicine at Mount Sinai
One G. Levy Place
New York, New York 10029
E-mail: isabelle.germano@mountsinai.org
Keywords: Fractionated radiotherapy, Gamma Knife, LINAC, proton beam, radiation, radiosurgery, vestibular schwannoma
Running title: Radiation Therapy and Radiosurgery for Vestibular Schwannomas
No part of this manuscript has been published or submitted for publication elsewhere
Abbreviations
CT: Computed tomography
GK: Gamma Knife
GR: Gardner–Robertson hearing scale
LINAC: Linear accelerator
MRI: Magnetic resonance imaging
NF2: Neurofibromatosis type 2
PTA: Pure tone average
SRS: Stereotactic radiosurgery
SRT: Stereotactic radiotherapy
VS: Vestibular schwannoma
Abstract
Radiosurgery versus Observation
Question
What are the indications for stereotactic radiosurgery (SRS) treatment versus observation for patients with intracanalicular vestibular schwannomas (VSs) without evidence of radiographic progression?
Target Population
This recommendation applies to all adults with VSs who have an imaging finding, such as magnetic resonance imaging (MRI) or computed tomography (CT), consistent with VSs without radiographic progression.
Recommendation
Level 3: If tinnitus is not observed at presentation, it is recommended that intracanalicular vestibular schwannomas and small tumors (<2 cm) without tinnitus be observed as observation does not have a negative impact on tumor growth or hearing preservation compared to treatment.
Radiosurgery Technology
Question
Is there a difference in outcome based on radiosurgery equipment used: Gamma Knife (GK) versus linear accelerator (LINAC)-based radiosurgery versus proton beam?
Target Population
This recommendation applies to all adults with vestibular schwannomas who are candidates for SRS treatment.
Recommendation
There are no studies that compare two or all 3 modalities. Thus, recommendations on outcome based on modality cannot be made.
Radiosurgery Technique
Question
Is there a difference in outcome based on the dose delivered?
Target Population
This recommendation applies to all adults with vestibular schwannomas who are candidates for SRS.
Recommendation
Level 3: As there is no difference in radiographic control using different doses, it is recommended that for single fraction SRS doses, <13 Gy be used to facilitate hearing preservation and minimize new onset or worsening of preexisting cranial nerve deficits.
Question
Is there a difference in outcome based on the number of fractions?
Target Population
This recommendation applies to all adults with vestibular schwannomas who are candidates for SRS.
Recommendation
As there is no difference in radiographic control and clinical outcome using single or multiple fractions, no recommendations can be given.
Radiographic Follow-Up, Retreatment, and Tumorigenesis after Radiosurgery
Question
What is the best time sequence for follow-up images after SRS?
Target Population
This recommendation applies to all adults with vestibular schwannomas who underwent SRS treatment.
Recommendation
Level 3: Follow-up imaging should be obtained at intervals after SRS based on clinical indications, a patient’s personal circumstances, or institutional protocols. Long-term follow-up with serial MRIs to evaluate for recurrence is recommended. No recommendations can be given regarding the interval of these studies.
Question
Is there a role for retreatment?
Target Population
This recommendation applies to all adults with vestibular schwannomas who show radiographic progression after radiosurgery treatment.
Recommendation
Level 3: When there has been progression of tumor after SRS, SRS can be safely and effectively performed as a retreatment.
Question
What is the risk of radiation-induced malignant transformation of vestibular schwannomas treated with SRS?
Target Population
This recommendation applies to all adults with vestibular schwannomas after SRS.
Recommendation
Level 3: Patients should be informed that there is minimal risk of malignant transformation of vestibular schwannomas after SRS.
Neurofibromatosis Type 2
Question
What are the indications for SRS in patients with neurofibromatosis type 2?
Target Population
This recommendation applies to all adults with vestibular schwannomas who have a diagnosis of neurofibromatosis type 2.
Recommendation
Level 3: Radiosurgery is a treatment option for patients with neurofibromatosis type 2 whose vestibular schwannomas are enlarging and/or causing hearing loss.
Introduction
Rationale
There is a growing body of evidence that VSs can be controlled by radiosurgery. However, at the appropriate time of treatment, the treatment modality (Gamma Knife [GK], linear accelerator [LINAC]-based, proton beam), scheme (single fraction, hypo- or hyperfractionation, or conventional fractionation), dose, and posttreatment follow-up is still a matter of debate. This guideline was created to provide guidance on the use of radiation therapy for these tumors based on the data present in the literature. As in most topics, the soundness and usefulness of this data varies depending on study design and how the data was collected.
Radiosurgery refers to delivery of high-dose radiation with high precision to a target. This can be accomplished using photon or proton therapy. The former uses gamma-rays emitted by 60Cobalt sources (GK) or x-rays emitted by a LINAC or a cyclotron, which uses heavy charged particles (generally proton or carbon ion). In addition to different sources, radiosurgery can be delivered in 1 or multiple treatments. Single-fraction radiosurgery is usually referred to as SRS. When the treatment is delivered in a few fractions (2–5), it is referred to as hypofractionation and, when multiple fractions are used, as stereotactic radiotherapy (SRT). The word “stereotactic” is often used in conjunction with radiosurgery and radiotherapy to signify the use of high-precision delivery of radiation using surgical techniques to achieve this precision without involving a surgical procedure. The word stereotaxis is derived from the Greek words stereos “3-dimensional” and taxis “orderly arrangements.” To accomplish such precision, a stereotactic frame was first used by Leksell to treat a VS.1 Subsequent advances in computer software and machine hardware have allowed for a similar degree of precision using “face masks” to immobilize the patient without the need for a rigid frame. This procedure is also known as “frameless” SRS as opposed to “framed” SRS when a frame is used. Finally, the dose delivered can have an impact on tumor control and potential side effects of the radiotherapy intervention.2
Objectives
This guideline focuses on summarizing the role of SRS on VS tumor control, ie, the lack of radiographic progression, its side effects, including new deficits and potential malignant transformation or tumorigenesis in patients with sporadic VSs and in patients with NF2, using different delivery technologies and techniques. In addition, it explores the necessary radiographic follow-up after SRS and the role of SRS for patients with VSs who show radiographic progression.
Methods
Writing group and questions establishment
After establishing VS management as a priority for guideline development, the Joint Tumor Section of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) and the Guidelines Committee of the Congress of Neurological Surgeons selected a multidisciplinary group of individuals to carry out this project. The entire group of individuals were screened for conflict of interest and then assembled into smaller groups by general components of management. These groups then agreed upon the main questions pertinent to these management components and shared them with the overall group for modification. The task force was divided into groups by management topic and proceeded with writing of the guidelines.
Search Method
A broad search strategy was used because of the relatively small number of studies on each specific topic. PubMed and the Cochrane Library were searched according to the strategy summarized in Table 1. The searches of electronic databases were supplemented with manual screening of the bibliographies of all retrieved publications. The bibliographies of recent systematic reviews and other review articles were also searched for potentially relevant citations. All articles identified were subject to the study selection criteria listed below. As noted above, the guideline committee also examined lists of included and excluded studies for errors and omissions. We went to great lengths to obtain a complete set of relevant articles. Having a complete set ensures that our guideline is not based on a biased subset of articles.
General Eligibility Criteria for Literature
General eligibility criteria were then applied with the resultant narrowing of the abstract publications as follows:
- Deduplication of references
- Limiting to human references
- Limiting to English references
- Limiting to January 1, 1946 to December 31, 2014
Article Inclusion and Exclusion Criteria
Abstracts for the initial 956 references were then reviewed and selected based on them meeting the following predetermined criteria:
General
- Investigated patients suspected of having VSs
- Was of humans
- Was not an in vitro study
- Was not a biomechanical study
- Was not performed on cadavers
- Was published between January 1, 1990 and December 31, 2014
- Was published in a peer-reviewed journal
- Was not a meeting abstract, editorial, letter, or commentary
- Was published in English
- Included quantitatively presented results
- Was not a review article
Specific
- Outcomes that included adult patients with VSs,
AND
- Outcomes following radiation therapy reported in ≥5 patients.
Figure 1 (PRISMA Diagram) summarizes the flow after the literature search.
Search Strategies
The task force collaborated with a medical librarian to search for articles published between January 1, 1990 and December 31, 2014. Two electronic databases, PubMed and the Cochrane Library were searched. Strategies for searching electronic databases were constructed by the evidence-based clinical practice guideline task force members and the medical librarian using previously published search strategies to identify relevant studies (Table 1 and Figure 1).
Classification of Evidence and Guideline Formulation
The concept of linking evidence to recommendations has been further formalized by the American Medical Association (AMA) and many specialty societies, including the AANS, the CNS, and the American Academy of Neurology (AAN). This formalization involves the designation of specific relationships between the strength of evidence and the strength of recommendations to avoid ambiguity. In the paradigm for therapeutic maneuvers, evidence is classified into that which is derived from the strongest clinical studies (eg, well-designed, randomized controlled trials), or Class I evidence. Class I evidence is used to support recommendations of the strongest type, defined as Level 1 recommendation, indicating a high degree of clinical certainty. Nonrandomized cohort studies, randomized controlled trials with design flaws, and case-control studies (comparative studies with less strength) are designated as Class II evidence. These are used to support recommendations defined as Level 2, reflecting a moderate degree of clinical certainty. Other sources of information, including observational studies such as case series and expert opinion, as well as randomized controlled trials with flaws so serious that the conclusions of the study are truly in doubt are considered Class III evidence and support Level 3 recommendations, reflecting unclear clinical certainty. A basis for these guidelines can be viewed at: here.
Results
Radiosurgery Treatment Versus Observation
Question 1
What are the indications for radiosurgery (SRS) treatment versus observation for patients with intracanalicular vestibular schwannomas without evidence of radiographic progression?
Target Population
This recommendation applies to all adults with an intracanalicular vestibular schwannomas who have an imaging finding, such as magnetic resonance imaging or computed tomography, consistent with vestibular schwannomas without radiographic progression.
Recommendation
Level 3: If tinnitus is not observed at presentation, it is recommended that intracanalicular vestibular schwannomas and small tumors (<2 cm) without tinnitus be observed as observation does not have a negative impact on tumor growth or hearing preservation compared to treatment.
Study Selection and Characteristics
A total of 47 studies were screened and assessed for eligibility, and 22 publications were included in the final review.3–24 Specific to this question only, studies reporting radiographic follow-up with MRI were included.
Items of interest for data extraction included study design, class of evidence, primary treatment modality, total number of patients, number of patients with lack of radiographic progression, study selection parameters, mean or median tumor size, mean or median follow-up, and inclusion of NF2.
Risk of Bias and Study Limitations
Because all the selected publications were retrospective or nonrandomized prospective studies, there is substantial risk of treatment selection bias. Currently, there is no evidence to determine if early treatment is beneficial. In some centers, all asymptomatic intracanalicular VSs might be treated “up front,” whereas in others they might not be treated until radiographic progression is documented. This can clearly bias the results obtained from this retrospective review. In addition, because age can have an effect on neurodegenerative changes, the decreased hearing after SRS/SRT might be a combined effect of the treatment and physiological aging. The two cannot be sorted out in the absence of a randomized, equipoised clinical trial.
Results of Individual Studies
VSs represent 8% of all primary brain neoplasms and approximately 16% of benign brain tumors.25 These tumors are usually slow growing, and most patients with small VSs have slight or imperceptible symptoms. An increasing number of VSs are detected incidentally by MRI for minor or unrelated symptoms.16,18 The timing of treatment of this type of tumor continues to be controversial. The key results of individual studies that provide information on natural history of untreated VSs are outlined in Table 2 and summarized within the guideline recommendations.
Growth Rate
The growth range in observational studies with follow-up of ≥2 years ranges from 13% to 74%. Growth patterns are not useful to predict need for treatment.21 Larger tumor size (14–20 mm) is a predictor of future growth.7,11,12,24 Regression in tumor size in the observational population was noted ranging from 10%14 to 12.5%.9
In 70 patients, the reported tumor growth rate in the first year was predictive of the growth rate in the second year.24 Larger tumors and those with a higher growth during the first year tended to grow faster. At the end of the 2 years, 61 patients did not require surgery (87%). Growth was 1.15 ± 2.4 mm/year,10 1.52 mm/year,20 and 1 mm/year.19
In 161 patients with radiographic increase in size, only 45% continued to grow.13 In a study with 47 patients and mean follow-up of 43.8 ± 40 months, 74% of patients showed growth compared to 3% treated with SRS. Tumors were not stratified by size.6 Another study with 47 patients7 and follow-up of 3.6 years showed a 37% tumor growth rate. In 180 patients, larger tumors at presentation had a higher chance of growing: each 1 mm increased the odds of growth by 20%.
Differences between Intra- and Extracanalicular Tumor Growth
In 73 patients, intracanalicular tumors were less likely to grow (7% vs 20%).3 Larger tumors (>20 mm) were also associated with an increased likelihood of growth. In 110 patients, 90% of intracanalicular tumors did not grow at 5 years, compared to 74% and 45% in larger tumors.14
Symptoms
Tinnitus worsened in the observational group (289 patients) compared to the intervention group (1138 patients) treated with surgery or SRS.26 Tinnitus at presentation increased the odds of tumor growth threefold.8 These authors raised the question that tinnitus may be a marker of increased biologic auditory nerve activity associated with tumor growth. Also, disequilibrium was more associated with patients that showed progressive growth.11
Useful hearing was preserved in 37% (60% of 161) of patients during the observation period with mean follow-up of 6.1 years.5 A study with 47 patients7 showed hearing preservation similar to the intervention group. Similar results were reported in a 239 patient study.4 In 636 prospectively allocated patients receiving conservative management, 88% still had good speech discrimination at 10- year observation.12 Hearing preservation occurred in 73% of 123 patients independent of growth.13
Synthesis of Results
Based on the studies above, if tinnitus is not reported at presentation, it is recommended that intracanalicular lesions should be observed prior to treatment. Small tumors (<2 cm) can be observed, as observation does not have a negative impact on tumor growth or hearing preservation compared to treatment. However, because tumor growth is more likely to be associated with observation than treatment, treatment might be required in patients undergoing observation. If tinnitus is present, the probability of growth is higher. In addition, tinnitus improves after SRS.
Discussion and Summary
A conservative approach is the preferred strategy for treatment of intracanalicular and tumors ≤2 cm sporadic incidental VSs. If this path is chosen, periodic monitoring with MRI is necessary to exclude growth.3,19 This is particularly important because there is no clear data to allow a true prediction of growth rate,17 although some studies suggest that tumor growth rate at 1 year is a predictor of future growth.23
The evidence for this guideline was primarily drawn from studies with Class III evidence. Currently, no Class I or Class II evidence exists to guide recommendations for this topic. These data should be used when counseling patients regarding the probability of observation when an incidental and asymptomatic sporadic VS is diagnosed on MRI. If tinnitus is present, the probability of growth rate is higher.
Radiosurgery Technology
Question 2
Is there a difference in outcome based on radiosurgery equipment used: Gamma Knife versus LINAC-based radiosurgery versus proton beam?
Target Population
This recommendation applies to all adults with vestibular schwannomas who are candidates for SRS treatment.
Recommendation
There are no studies that compare 2 or all 3 modalities. Thus, recommendations on outcome based on modality cannot be made.
Study Selection and Characteristics
A total of 538 studies were screened and assessed for eligibility, and 48 publications were included in the final review, specifically 33 for GK,27–59 11 for LINAC,60–70 and 4 for proton beam.71–74 Specific to this question, only studies reporting on patients treated with GK, LINAC, or proton beam radiosurgery with follow-up MRI and clinical outcome were included. Outcome was defined as radiographic control and lack of new deficits, including hearing preservation, trigeminal and facial function, and other neurological deficits as reported. Data extraction included study design, class of evidence, primary treatment modality, total number of patients, number of patients with lack of radiographic progression, study selection parameters, mean or median tumor size, mean or median follow-up, inclusion of NF2, percentage of patients with serviceable hearing, percentage of patients with new onset of cranial nerve neuropathy (facial or trigeminal or other), and percentage of patients with new other deficit. Articles before 1996 were not included in evidence tables because it became obvious that differences in dosing had a significant impact on functional outcome, as will be discussed in the following paragraphs. When the same author presented series in different years, the latest one or the one with the largest number of patients was included in this review.
Risk of Bias and Study Limitations
As all selected publications were retrospective or nonrandomized prospective studies, there is substantial risk of treatment selection bias. For example, some centers might not treat intracanalicular lesions until radiographic progression is documented, whereas others treat more aggressively. Since this is not always specified in the methods, there might be lack of equipoise when comparing modalities. In addition, given that dose might have an impact on outcome, an attempt to control for variance in radiation planning parameters was made. Finally, lack of reporting of side effects other than cranial nerve deficits could represent a bias in the sense that lack of reporting might not mean lack of observation, but perhaps “omission” as outside of the scope of the report. This comment might be relevant to the observation that hydrocephalus was reported only in GK and proton beam series and not in LINAC (see below). The degree of the deficit is also important as some authors only report permanent deficits while others combine temporary with permanent.
Results of Individual Studies
The key results of individual studies are summarized in Tables 3A, 3B, and 3C.
Tumor Control
There are no differences in radiographic control comparing series treated with GK versus LINAC-based therapy. Radiographic control ranged from 100%60 to 88.5%61 in LINAC-based series, and 100%45,58 and 71%38 in GK series. Tumor control rates decreased regardless of the technology used with longer follow-up.42–44,47 Tumor size had an impact on radiographic control, with smaller tumors (<3 cm) showing the highest tumor control rate at comparable time intervals, regardless of the technology used.33 Similarly,50 reported higher tumor control with tumor volumes <10 cc3.
Notably, several authors describe a transient tumor volume enlargement within the first 2 years of SRS with subsequent stabilization or decrease.48,57,75–78 Awareness of this fact is necessary to avoid performing surgery within 6 months of treatment, as reported by Yang et al.79 Additional discussion of this aspect is presented in a different section of these guidelines.
Proton beam series are less numerous and seem to have a similar control rate, with noted tumoral decrease with longer follow-up. For example,74 reported a radiographic control of 94% at 2 years followed by 84% at 5 years.
Clinical Outcome: Hearing Preservation and Side Effects
There were no differences in clinical outcome comparing series treated with GK versus LINAC-based therapy when considering hearing preservation or new deficits to cranial nerves VII and V. Similar to radiographic control, hearing preservation decreased with longer follow-up regardless of the technology used. Combs et al63 reported a hearing preservation of 90% at 1 year, decreased to 69% at 10 years using LINAC-based technology. Similarly, Hasegawa et al39 using a GK, reported a decrease in hearing preservation from 54% at 3 years to 34% at 8 years. In addition, regardless of the technology used, there are data supporting the concepts that cochlear spearing, higher auditory function at baseline, and young age can all favorably contribute to higher rates of hearing preservation after SRS. Hasegawa et al39 reported that in patients receiving <4 Gy to the cochlea, hearing preservation at 3 years was 80% and 70% at 8 years (in contrast to 55% and 34%, respectively, with higher cochlear dose). Bashnagel et al80 reported a cochlear dose <3 Gy to have favorable prognostic outcome on hearing preservation. Boari et al27 reported the highest hearing preservation in patients <55 years of age with Gardner–Robertson (GR) Class 1 hearing prior to SRS, 93% compared to 71% in patients >55 years of age, and to 49% for the overall population, independent of GR class and age. Similarly, Franzin et al41 associated GR Class 1 hearing and age <54 years old as favorable prognostic factors for hearing preservation. Lundsford31 noted that hearing preservation is higher in patients with intracanalicular VSs.
Complication rates for facial and trigeminal cranial nerve deficits were similar for LINAC and GK radiosurgery. In most series, the rate of trigeminal neuropathy was greater than that of facial neuropathy (Table 3).36,49,51,53,59,64,67,68 Two studies reported facial nerve deficits greater than trigeminal.66,69 In series with a dose ≤13 Gy, new facial nerve deficits were reported in ≤11% of patients treated with GK50 and 5% of patients treated with LINAC-based technology.67 New trigeminal nerve deficits occurred in up to 11.7%34 of patients treated with GK50 and 11% of patients treated with LINAC-based technology.65 New onset of cranial nerve neuropathy was associated with higher tumor volume (>3 cm).68 Kondziolka et al59 observed that complete facial paralysis occurred only in patients who had a preexisting 7th cranial nerve deficit.
Proton beam series had similar radiographic control but substantially lower hearing preservation rates.73,74
Independent of the delivery modality, the dose delivered made a difference in outcome for both preservation of function (hearing preservation) and avoidance of new deficits (facial weakness and numbness). As summarized in Tables 3A and 3B, doses ≤13 Gy maintained excellent tumor control while minimizing side effects. Finally, there was consensus that new cranial nerve side effects were unlikely to occur after 96 months (8 years).
Hydrocephalus after SRS was only reported in GK- and proton beam–treated patients with a rate up to 16%.32 In addition, in a review paper, Han et al81 had previously reported a hydrocephalus rate of 5.6 % in 444 patients with sporadic VSs treated with GK radiosurgery.
Other presenting symptoms showed variable outcome after SRS. Tinnitus was found to improve from 52% to 28% by Gerosa et al.40 On the other hand, Boari et al27 reported that it never improved after SRS. Gait/balance and vertigo improved 25%63 and 30%.40 The same symptoms were described to newly occur after SRS: tinnitus at 13% and gait/balance/vertigo at 14%.82 Murphy83 reported new onset of vertigo in 4% and gain imbalance in 18% of patients with VSs treated with SRS.
Synthesis of Results
The reviewed data show similar radiographic control comparing series treated with GK versus LINAC-based therapy. However, there are no studies comparing directly these 2 modalities. Tumor control rates decreased regardless of the technology used with longer follow-up. At 10 years, reported radiographic control ranges from 91%46 and 65.7%.29 There are no differences in clinical outcome comparing series treated with GK versus LINAC-based therapy when considering hearing preservation or new deficits to cranial nerves VII and V. Similar to radiographic control, hearing preservation decreased with longer follow-up regardless of the technology used. Proton beam series are less frequent; however, they compare favorably with GK and LINAC for radiographic control. Hydrocephalus was reported after GK and proton beam SRS but not LINAC SRS. However, no study directly compared these different technologies regarding this side effect.
Discussion and Conclusion
A full review of basic radiosurgery principles using LINAC, GK, and proton beam radiosurgery is beyond the scope of this work and can be found elsewhere.2 Since hearing preservation declines with longer follow-up, some investigators have attributed this observation to the effect of normal aging rather than delayed effects of SRS.63 An identified predicting factor for hearing preservation was identified as initial pure tone average (PTA) >20 dB with 5 times greater than normal change of decreased hearing over time compared to patients with PTA <20 dB. In addition, GR Class 1 hearing was associated with higher hearing preservation.27 The authors suggest that on this basis, patients with good baseline hearing should undergo SRS sooner to maximize their hearing preservation opportunity.
Of note, all 3 proton beam series using single fraction were >10 years old. Factors that might explain this observation include the fact that proton beam equipment requires a much larger physical plant and infrastructure. In addition, because the hearing preservation rate was lower than the other 2 technologies, it is possible that physicians preferentially treated VS patients using GK or LINAC.
Radiosurgery Technique
Question 3
Is there a difference in outcome based on the dose delivered?
Target Population
This recommendation applies to all adults with vestibular schwannomas who are candidates for SRS.
Recommendation
Level 3: As there is no difference in radiographic control using different doses, it is recommended that for single fraction SRS doses, <13 Gy be used to facilitate hearing preservation and minimize new onset or worsening of pre-existing cranial nerve deficits.
Question 4
Is there a difference in outcome based on the number of fractions?
Target Population
This recommendation applies to all adults with vestibular schwannomas who are candidates for SRS.
Recommendation
As there is no difference in radiographic control and clinical outcome using single or multiple fractions, no recommendations can be given.
Study Selection and Characteristics
A total of 202 studies were screened and assessed for eligibility, and 15 publications were included in the final review (6 for question 375,84–88 and 9 for question 463,82,89–95). Specific to these questions, only studies reporting radiographic follow-up with MRI were included.
Data extraction included study design, class of evidence, primary treatment modality, total number of patients, number of patients with lack of radiographic progression, study selection parameters, mean or median tumor size, mean or median follow-up, and inclusion of NF2.
Risk of Bias and Study Limitations
Because all selected publications were retrospective or nonrandomized prospective studies, there is substantial risk of treatment selection bias. Finally, significant selection bias exists in selection of a fractionation scheme other than single fraction. Variations in radiation doses prescribed, prescription isodose selected, dose homogeneity, and variation in treatment planning techniques need to be considered. Reported data may also be difficult to interpret because of variation in terminology used to report varying fractionated schemas, particularly when referring to SRT, hypofractionation, and “standard” external beam irradiation.
Results of Individual Studies
Dose
With respect to the dose delivered for treatment of VSs, the literature was largely comprised of Level III evidence (Table 4). Widespread variations in dose delivered to VSs have been reported. For SRS or SRT, a lower dose appeared to confer a greater chance for preservation of neurological function provided of course that the tumor was controlled. Based upon short to intermediate follow-up periods, hearing and facial nerve function were more likely to be preserved with a lower dose as compared to a higher one within the therapeutic range described in the literature.75,84 However, within the range of doses used for the treatment of VSs, a lower dose had little to no appreciable difference in progression-free survival, and generally high rates of progression-free survival were reported across a wide range of delivered doses.75,84,86,88 Based upon the currently available evidence, an optimal dose for single-fraction SRS, hypofractionated SRS, or SRT cannot be ascertained. Further clinical investigation will be required.
Fraction Numbers
Evidence comparing the various fractionation techniques comprise Level III (Table 5). SRS has typically been used for tumors ≤3 cm in diameter, whereas other techniques have been used for larger tumors, thereby making the study cohorts dissimilar and comparison of clinical outcomes between disparate cohorts problematic.88,89,91,94,96 High rates of progression-free survival (ie, generally ≥90%) were afforded by single fraction, hypofractionated, or traditional fractionated schemes.33,84,97 As compared to tumor control, lower rates of hearing preservation were reported, and hearing preservation rates lessened with longer follow-up assessment and for larger tumors.39,50 Rigorous evidence supporting a single fraction approach, compared to others for preserving hearing, seems lacking.63,90 Further clinical investigation will be required to determine an optimal fractionation approach for VS patients. However, a one-size-fits-all approach is not likely to be ascertained, and an optimal approach may vary based upon various factors, including tumor size (or volume) and neurologic function for particular patient cohorts at the time of presentation for treatment.
Synthesis of Results
Based on the studies discussed above, there is no significant difference in radiographic control using doses ≤13 or >13 Gy for SRS. There is improved hearing preservation and decreased side effects defined as a new cranial nerve deficit using doses <13 Gy. Therefore, Class III evidence supports that a dose of ≤13 Gy should be used. Data on hypofractionated SRS and SRT were too heterogeneous to allow for a conclusion on the recommended dose or fractionation scheme. There is no recommendation that can be given based on the available data regarding the schemes of the fractionation and which patient population will benefit from that. Hearing preservation rates lessened with longer follow-up assessment and for larger tumors regardless of the treatment scheme used. Overall, SRT studies suggest a slightly more favorable range of hearing preservation rate than SRS.
Discussion and Summary
Treatment planning for VSs is challenging because of their shape and their proximity to brain stem, cochlea, and other cranial nerves. The goal is to choose a technique that provides radiographic control while sparing tissue at risk. This review of the literature provides Class III evidence that a dose of ≤13 Gy will result in a reasonable rate of tumor control while lessening potential side effects like decreased hearing and increased cranial nerve deficits.
Another important point when choosing the best technique to treat VSs resides on the avoidance of organs at risk. While brain stem and cranial nerves are recognized as such, the cochlea is still a matter of debate. The first publication on the importance of the cochlea dose to hearing preservation after GK surgery for VSs was by Massager et al.98 In their retrospective study of 82 patients treated with a fixed margin dose of 12 Gy, they reported a mean cochlea dose of 4.33 Gy (range 1.30–10 Gy). Unlike other previous publications, they measured the mean cochlea dose averaged over the whole 3D volume of the cochlea and found that those with preserved hearing had a mean cochlea dose of 3.7 Gy versus 5.33 Gy in those who lost useful hearing. In another study comprising 69 patients treated for sporadic VSs using GK surgery, mean maximal dose to the cochlea was reported at 10.27 Gy (range 3.1 Gy–16.1 Gy).99 The study authors have claimed that significant relations exist between the maximal cochlea dose and the difference in the PTA before and after GK surgery. Although no threshold has been suggested, the authors emphasized the need for exact radiation planning to reduce the cochlea radiation dose if the hearing is to be preserved.
In conclusion, the evidence for this guideline was primarily drawn from Class III studies. A Level 3 recommendation stands to use a dose of ≤13 Gy to achieve radiographic control while minimizing adverse effects should be used while planning SRS for VSs. Until more robust data are available, decreasing the dose to the cochlea while planning for SRS should be kept in mind, while not compromising tumor dose. Patients should be counseled about the lack of evidence supporting single fraction, hypofractionated SRS, or SRT while being reminded that the hearing preservation range is slightly higher with SRT. Hearing preservation rates lessened with longer follow-up assessment and for larger tumors regardless of the treatment scheme used.
Radiographic Follow-Up, ReTreatment, and Tumorigenesis after SRS
Question 5
What is the best time sequence for follow-up images after radiosurgery?
Target Population
This recommendation applies to all adults with vestibular schwannomas who underwent SRS treatment.
Recommendation
Level 3: Follow-up imaging should be obtained at intervals after SRS based on clinical indications, a patient’s personal circumstances, or institutional protocols. Long-term follow-up with serial MRIs to evaluate for recurrence is recommended. No recommendations can be given regarding the interval of these studies.
Question 6
Is there a role for retreatment?
Target Population
This recommendation applies to all adults with vestibular schwannomas who show radiographic progression after radiosurgery treatment.
Recommendation
Level 3: When there has been progression of tumor after SRS, SRS can be safely and effectively performed as a retreatment.
Question 7
What is the risk of radiation-induced malignant transformation of vestibular schwannomas treated with SRS?
Target Population
This recommendation applies to all adults with vestibular schwannomas after SRS.
Recommendation
Level 3: Patients should be informed that there is minimal risk of malignant transformation of vestibular schwannomas after SRS.
Study Selection and Characteristics
For question 5, a total of 96 studies were screened and assessed for eligibility, and 8 publications77,78,94,100–104 were included in the final review. Specific to this question, only studies reporting radiographic follow-up with MRI were included. For question 6, 4 full-text articles were screened and assessed for eligibility, and 1 was excluded. (The excluded paper related to patients who underwent retreatment with surgical resection, not SRS.) Therefore, 3 articles were included.105–107 For question 7, 6 full-text articles were reviewed, and 4 were excluded (3 studies were case reports, and therefore were excluded, and 1 study addressed the development of VSs after treatment for other tumors). Therefore, 2 studies were identified and reviewed.84, 108
Data extraction included study design, class of evidence, primary treatment modality, total number of patients, number of patients with lack of radiographic progression, study selection parameters, mean or median tumor size, mean or median follow-up, inclusion of NF2, development of malignancy, and retreatment.
Risk of Bias and Study Limitations
Because all selected publications were retrospective or nonrandomized prospective studies, there is substantial risk of treatment selection bias. Pertinent to questions 6 and 7, the paucity of studies on the topic can add an additional source of publication bias in the sense that the reported number of cases on this topic might be underestimated. In addition, there should be a recognition that in the data collected in this retrospective manner, correlation does not imply causation. A second malignancy is generally a late effect. The difficulty in accurate, long-term follow-up may underestimate the risk of malignancy developing because of treatment.
Results of Individual Studies
Imaging Follow-Up
Table 6 summarizes these results. Follow-up imaging provides important information on the treatment effect of VS SRS. In all series analyzing VS treatment response after SRS, MRI was the imaging modality used to define tumor response. None of the studies reviewed had as its primary focus to determine the best posttreatment follow-up scheme. Follow-up MRI was an eligibility criterion for this question; therefore, all studies had ≥1 MRI after treatment. All studies have a follow-up MRI at 12 months after treatment. During the first year after SRS, follow-up intervals included MRIs every 3 to 4 months77,100,104,109,110 to every 6 months.111,112 During the second year after SRS/SRT, follow-up varied from 3 to 4 months to every 6 months.48,79,113–115
After year 5, Meijer et al94 followed their patients with yearly MRIs, whereas other studies followed their patients with 2-year intervals.59,101
Indications for Retreatment
Three studies were identified that specifically addressed retreatment with SRS after initial SRS treatment for VSs (Table 7). All studies are limited by their retrospective nature and small sample sizes, with a cumulative total number of 43 patients.
Kano et al105 retrospectively reviewed 6 patients who underwent initial SRS and subsequently had imaging evidence of tumor progression. All patients were retreated with SRS after a median time of 63 months. Patients received a median margin dose of 11 Gy. At median 29-month follow-up, 2 of 6 (33.3%) patients had tumor control (ie, no further progression), and 4 of 6 (66.7%) patients had tumor regression. No patients had adverse radiation effects or new neurological symptoms. Liscak et al106 retrospectively reviewed 24 patients treated with GK surgery who showed progression (defined as 2 mm growth and enlargement that persisted for 2 years after treatment). Original treatment was with a median dose of 12.5 Gy (at median 50% isodose). Patients were retreated with a median dose of 13 Gy (at median 50% isodose). Twenty-two of 24 patients (91.7%) showed regression or control of tumor progression. Overall, 4 (16.7%) patients experienced new neurologic symptoms, including 1 patient with worsening facial function, 2 patients with trigeminal neuropathy, and 1 patient with vertigo. Dewan et al107 retrospectively reviewed 11 patients previously treated with SRS (10 patients with GK surgery and 1 patient with proton beam therapy), who experienced tumor progression at a mean time from first treatment of 51 months. The initial prescription dose used for GK surgery was 12 Gy (at 50% isodose line). The initial prescription dose used for proton beam therapy was 13.2 Gy (at 77% isodose line). Retreatment was with a median of 12 Gy (at a median 50% isodose). Nine of eleven (81.8%) patients experienced a decrease in tumor or size or control of tumor growth after retreatment. One out of eleven patients experienced progression requiring surgical resection 6 years later. Four patients experienced new or worsening neurologic symptoms, which included 2 patients with facial numbness and tingling, one patient with decreased hearing (Class I to II), and 1 patient with significant radiation-induced edema resulting in headaches and vertigo.
Risk of Malignant Transformation or Tumorigenesis
There are 13 cases reported of radiation-induced malignancies in patients harboring VSs treated with radiosurgery.20,21,50,79,116–124 There are at least 9 cases of radiation-induced malignant peripheral nerve sheath tumor, which appears to be the most common tumor type in this category. Other reported tumor types include meningiosarcoma, glioblastoma multiforme, Triton tumor, high grade undifferentiated sarcoma, and pleomorphic sarcoma. The true rate of malignant transformation in VSs is unknown. There were only 2 studies that fit the search criteria and addressed the question of the risk of radiation-induced malignant transformation of VSs (Table 8). Rowe et al108 retrospectively assessed the safety of radiosurgery in 137 patients with NF2 and von Hippel–Lindau disease. A total of 146 VSs were treated with radiosurgery. Two patients experienced suspected malignant transformation. The first patient had a rapidly growing VS that was treated by radiosurgery with 15 Gy to the prescription isodose. Three years later, the lesion was resected because of progression. Histologic analysis revealed “malignant transformation” in a schwannoma. The second patient had a VS treated with 14 Gy to the margin. Three years after treatment, the patient developed a glioblastoma. The authors provided their opinions regarding causality with respect to treatment with SRS and malignant transformation in these 2 patients. Details of the exact relation of the glioblastoma and the schwannoma are not available. In the first patient, the authors believe the tumor was exhibiting “atypical behavior” before radiosurgery and that the growth pattern was unchanged after radiosurgery, suggesting this was not a condition of malignant transformation but rather a primary malignant nervous system tumor. In the second patient, the authors stated that approximately 4% of NF2 patients develop gliomas, and it is unclear if radiation increased the risk of malignant transformation. The second study by Hasegawa et al84 was a retrospective review of 440 patients with VSs who were treated with GK surgery. Three hundred forty-seven patients (79%) underwent GK surgery as an initial treatment and 93 patients (21%) underwent GK surgery after microsurgical resection. Patient follow-up duration was for a median of 12.5 years. One patient experienced malignant transformation at 66 months. The patient had a resection at 52 months for tumor progression, although histologic analysis revealed that it was a benign tumor. The tumor recurred a second time and underwent a repeat resection at 66 months. Histologic analysis of that second specimen revealed malignancy. The overall malignant transformation rate observed in this analysis was 0.3%, and the annual incidence of malignant transformation was 0.02%.
Synthesis Of Results
Class III evidence supports that after radiosurgery magnetic resonance images are indicated to determine tumor control. During the first year, most studies document the use of ≥2 MRIs with some documenting MRI follow-up every 3 months. During years 2 to 5, most studies documented yearly or biannual follow-up. After 5 years, some authors are following patients every other year or even less often. Class III evidence supports that retreatment after radiosurgery in patients with radiographic progression results in tumor control with favorable outcome.
Class III evidence supports there is minimal risk of malignant transformation of VSs or tumorigenesis after SRS/SRT.
Discussion and Summary
In conclusion, after SRS/SRT, the recommendations of this guideline for imaging follow-up, retreatment, and tumorigenesis is based on Class III evidence.
Magnetic resonance images are indicated to determine tumor control. During the first year, follow-up schemes vary from every 3 months to every 6 months to once per year. During years 2 to 5, most studies documented yearly or biannual follow-up. After 5 years, the authors reported performing radiographic control yearly, every other year, or even less frequently. Long-term follow-up with serial MRIs to evaluate for recurrence is recommended. No recommendations can be given regarding the interval of these studies.
When tumor progression occurs after initial treatment with SRS/SRT, a second SRS/SRT treatment appears to provide good tumor control without major adverse treatment effects, based on a modest number of small, retrospective studies. Larger, prospective studies or prospective clinical data base are necessary to further address the safety and efficacy of a second SRS/SRT treatment with documented tumor progression.
Though it is a relatively rare phenomenon, radiation-induced malignant transformation of VSs have been reported in the literature. The true incidence of malignant transformation is unknown, although Hasagewa et al84 suggest an overall malignant transformation rate of 0.3% and an annual incidence of 0.02%. Long-term studies are necessary to identify at-risk patient populations, and patients should be informed of this rare but life-threatening complication before radiosurgery.
Radiosurgery in patients with NF2
Question 8
What are the indications for SRS in patients with neurofibromatosis type 2?
Target Population
This recommendation applies to all adults with vestibular schwannomas who have a diagnosis of neurofibromatosis type 2.
Recommendation
Level 3: Radiosurgery is a treatment option for patients with neurofibromatosis type 2 whose vestibular schwannomas are enlarging and/or causing hearing loss.
Study Selection and Characteristics
A total of 26 studies were screened and assessed for eligibility, and 15 publications were included in the final review.125–139 Specific to this question, only studies that reported radiographic follow-up with MRI and patients with NF2 were included.
Risk of Bias and Study Limitations
Because all the selected publications were retrospective or nonrandomized prospective studies, there is a substantial risk of treatment selection bias. Many institutions preferentially manage NF2 patients with surgery, so there is a potential bias in the selection of patients. Also, VSs in neurofibromatosis can occur in younger patients and are not infrequently bilateral, which may lead to a biased sample of patients treated with SRS. In addition, the smaller number of patients in each retrospective study can induce a further source of publication bias in the sense that the reported number of cases might be underestimated. Finally, there should be recognition that in retrospectively collected data, correlation does not imply causation.
Results of Individual Studies
The use of SRS for treatment of VSs in NF2 patients has become an important treatment option mainly because of low cranial nerve morbidity (hearing loss and facial nerve dysfunction) and good tumor control. A total of 15 (Table 9) retrospective, single-institution studies have analyzed the role of SRS in management of VS tumors in NF2 patients. These series found hearing preservation was less than in NF2 patients than in patients with sporadic VS tumor undergoing SRS. Tumor control rates in 1 series were 85%, 81%, and 81% at 5, 10, and 15 years after SRS treatment, respectively.134 Rowe et al135 found that in 122 VS tumors treated with SRS, there was 50% local control of the tumor after 8 years. Despite having less HN preservation and tumor control rates than sporadic VS tumors treated, SRS still is an important treatment option for patients with NF2 and VS tumors that may be enlarging and causing hearing loss.
Synthesis of Results
Class III evidence supports the use of SRS as primary management for VS tumor control and hearing preservation in NF2 patients, who are symptomatic with enlarging tumors. Class III evidence shows that VS tumor control and hearing preservation in NF2 patients after SRS may not be as effective as SRS treatment of sporadic VS tumors. Class III evidence supports observation of VS tumors in asymptomatic NF2 patients with no tumor enlargement. Class III evidence supports low facial nerve neuropathies after SRS treatment of VS tumors in NF2 patients.
Discussion and Conclusion
Based on Class III evidence, SRS is a treatment option for symptomatic NF2 patients with enlarging VS tumors. Good tumor control and hearing preservation are possible with SRS treatment of VS tumors in NF2 patients at 5 years. However, VS tumor control and hearing preservation rates are lower in NF2 patients in comparison to sporadic VS tumors after SRS treatment. Preservation of facial nerve function can be routinely possible after SRS treatment of NF2 VS tumors. In NF2 patients who are asymptomatic with no VS tumor enlargement, continued observation is preferred.
Key Issues for Future Investigation
As stated throughout this paper, the evidence-based data is derived from Class III studies. It would be desirable to construct prospective and randomized clinical trials aimed at increasing the evidence levels for each of the posed questions. However, it is unlikely that a prospective randomized trial comparing outcomes among different equipment will ever materialize because there are a significant number of obstacles, including the fact that most centers would only have one type of equipment. VSs remain a relatively uncommon tumor with a less than clearly defined natural history, which makes patient enrollment and clinical equipoise challenging for randomized clinical trials. In addition, it is most likely that the enrollment numbers required to detect clinically meaningful differences would require a high number of patients, thus necessitating a long time during which technology and technique could change. Finally, by the time long-term data have been acquired, the state of the field may have changed significantly because of improvements in radiation treatment paradigms.
Nonetheless, higher levels of evidence are required to better define clinical outcomes and best practices. National and international prospective quality registries for VS patients managed with SRS and other approaches (ie, observation and microsurgery) may prove more effective in generating the information that is needed to answer important clinical questions that remain. One such registry is currently accruing patients in a multicentric fashion in the United States. This national registry, which is a joint effort of the American Society for Radiation Oncology (ASTRO), the AANS, and the CNS, will define national patterns of care in radiosurgery, with a focus toward improving health care outcomes, supporting informed decision making, and potentially lowering the cost-of-care delivery to patients.
Technological upgrades to SRS and SRT devices may also advance the treatment of VSs. Advanced imaging such as diffusion tensor imaging techniques to account for fiber tracts is now being integrated into dose planning. The implications of dose to lengths or volumes of these tracts and the differential response of such tracts warrant investigation. Interfractional adaptive planning for hypofractionated SRS and onboard low or standard frequency MRI for cobalt and linear accelerator-based SRS devices are being applied to intracranial radiosurgery. These refinements may help to improve clinical outcomes for patients afflicted with VSs.
Conflict of Interest (COI)
The Vestibular Schwannoma Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript (here).
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Committee for its review, comments, and suggestions throughout peer review, as well as Mary Bodach, MLIS, for her assistance with the literature searches. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Sepideh Amin-Hanjani, MD, D. Ryan Ormond, MD, Andrew P. Carlson, MD, Kimon Bekelis, MD, Stacey Quintero Wolfe, MD, Chad W. Washington, MD, Cheerag Dipakkumar Upadhyaya, MD, and Mateo Ziu, MD
Figures
Figure 1. PRISMA flow chart.
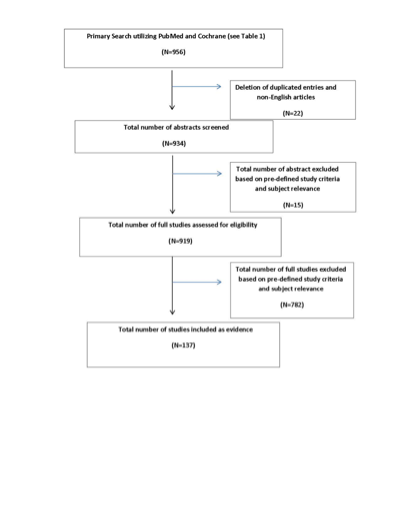
Table 1. Primary search strategy
|
PUBMED (NLM), searched on April 13, 2015:
|
|
Step 1: Neuroma, Acoustic [MeSH]
|
|
Step 2: (vestibular [Title/Abstract] OR vestibulocochlear [Title/Abstract] OR acoustic [Title/Abstract]) AND (neuroma* [Title/Abstract] OR neurilemmoma* [Title/Abstract] OR neurilemoma* [Title/Abstract] OR neurinoma* [Title/Abstract] OR tumor* [Title/Abstract] OR tumour* [Title/Abstract] OR schwannoma* [Title/Abstract])
|
|
Step 3: Step 1 OR Step 2
|
|
Step 4: Radiotherapy [MeSH] OR Radiotherapy [SH]
|
|
Step 5: Radiosurg* [TIAB] OR radiother* [TIAB] OR radiation therap* [TIAB] OR gamma knife [TIAB] OR cyberknife [TIAB] OR linac [TIAB] OR brainlab [TIAB] OR proton beam [TIAB] OR stereotact* [TIAB] OR stereotaxi* [TIAB] OR SRS [TIAB]
|
|
Step 6: Step 4 OR Step 5
|
|
Step 7: Step 3 and Step 6
|
|
Step 8: Step 7 AND English [Lang]
|
|
Step 9: (animal [MeSH] NOT human [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR case reports [PT]
|
|
Step 10: Step 8 NOT Step 9
|
|
Step 11: Step 10 AND (“1946/01/01” [PDAT] : “2015/01/01” [PDAT]
|
|
Total: 925 Results
|
|
COCHRANE, searched on April 13, 2015:
|
|
Step 1: MeSH descriptor: [Neuroma, Acoustic] explode all trees
|
|
Step 2: ((vestibular or vestibulocochlear or acoustic) and (neuroma* or neurilemmoma* or neurilemoma* or neurinoma* or tumor* or schwannoma*)):ti,ab,kw
|
|
Step 3: Step 1 OR Step 2
|
|
Step 4: MeSH descriptor: [Radiotherapy] explode all trees
|
|
Step 5: Any MeSH descriptor with qualifier(s): [Radiotherapy - RT]
|
|
Step 6: Radiosurg* or radiother* or radiation therap* or "gamma knife" or cyberknife or linac or brainlab or "proton beam" or stereotact* or stereotaxi* or SRS:ti,ab,kw
|
|
Step 7: Step 4 or Step 5 or Step 6
|
|
Step 8: Step 3 and Step 7
|
|
Step 9: Filtered 1946-12/31/2014
|
|
Total: 31 Results
|
|
Summary of Primary Search
Combined from 2 database searches, total of 956 candidate articles
|
Table 2. Observation versus radiosurgery/radiation treatment in vestibular schwannoma patients
|
Author/Year
|
Study Design
|
Class of Evidence
|
Study Conclusions Specific to Questions
|
|
Gonzalez-Orus Alvarez-Morujo et al, 2014
|
Retrospective study of 73 VS patients followed conservatively with average tumor size 11.9 mm, 59% intracanalicular, mean follow-up 3.1 years.
|
III
|
Radiographic control reported at 88%; 12% increased in size (growth defined as 2- dimensional increase of ≥2 mm). The average growth rate = 0.62 mm/year. Intracanalicular tumors less likely to grow (7% vs 20%); 9.5% experienced change in symptoms; factors predicting growth included: change in initial symptoms; tumors associated with tinnitus, instability and sudden deafness at initial diagnosis; size (>20 mm); tumors with cystic component.
|
|
Breivik et al, 2013
|
Retrospective study of 239 VS patients: 124 managed conservatively and 113 treated with GK SRS; median follow-up 5.7 years; tumor volume <2.5 cm; marginal dose 12 Gy.
|
III
|
Serviceable hearing rate was 64% in GK SRS patients compared to 76% in conservative management. This difference was not significant.
|
|
Ferri et al, 2013
|
Retrospective study of 161 VS patients followed with serial MRIs every 6 months and audiogram; mean follow-up 6.1 years; tumor growth defined as >2 mm
|
III
|
In patients with radiographic increase in size who continued to be observed, only 45% continued to grow over time. 60% of patients with useful hearing at diagnosis preserved it during observation period. In some patients with documented growth, a “wait and scan” approach may be reasonable as less than half of these continued to grow.
|
|
Regis et al, 2013
|
Retrospective study of 47 VS patients; mean follow-up was 44 ± 40 months followed conservatively compared to 34 VS patients treated with SRS.
|
III
|
74% of “wait and see” group required treatment. Treatment failure in the SRS group was 3%. Hearing preservation rates in “wait and see” group were 75%, 52%, and 41% and in the SRS group 77%, 70%, and 64% at 3, 4, and 5 years. Authors concluded that “wait and see” exposes patients to higher risk of tumor growth and hearing degradation.
|
|
Pennings et al, 2011
|
Retrospective study of 47 VS patients all unilateral managed conservatively followed with MRI and audiogram; mean follow-up 3.6 years; tumor growth defined as >2 mm
|
III
|
Overall 74% of patients with good hearing (according to 50/50 rule, aka combination of PTA and WRS) maintained hearing above this rule. Observation hearing preservation outcomes yield results comparable to surgery or SRS. There was no significant difference in hearing loss between 3 subsites in the IAC (porus, fundus, and central). 37% of patients demonstrated tumor growth over a mean follow-up of 32 months.
|
|
Agrawal et al, 2010
|
Retrospective study of 180 VS patients all unilateral managed conservatively; tumor growth defined as >2 mm.
|
III
|
Larger tumor size at diagnosis associated with higher odds of tumor growth (each 1-mm increment in tumor size at presentation increased odds of growth by 20%). Tinnitus at diagnosis significantly increased odds of tumor growth, 3 times increase. Authors conclude that for patients of all ages, a period of observation during which tumor growth and hearing thresholds are closely monitored is the superior strategy.
|
|
Whitehouse et al, 2010
|
Retrospective study of 88 VS patients managed conservatively; average follow-up: 3.65 years; average tumor size: 11 mm.
|
III
|
Tumor control was observed in 49%: 13% decreased in size and 36% was stable. 25% failed conservative management and required treatment. Size at diagnosis (P = .037) and growth during first year of follow-up (P = .005) were significantly found to predict active intervention. Authors suggest that growth during the first year of follow-up should be considered in determining whether to recommend treatment.
|
|
Bakkouri et al, 2009
|
Retrospective study of 325 unilateral VS patients managed conservatively for >1 year. MRI repeated 1 year after diagnosis and then every 1–2 years depending on new symptoms or tumor growth.
|
III
|
Overall mean tumor growth was 1.15 ± 2.4 mm/year. 12% showed tumor growth >3 mm; 58% showed tumor growth rate <1 mm per year. The growth rates of intrameatal and extrameatal tumors did not differ significantly. Results support role of conservative management for small sized VS as majority demonstrate slow growth rate.
|
|
Malhotra et al, 2009
|
Retrospective study of 202 unilateral VS patients managed conservatively for mean 2.48 years.
|
III
|
9.4% patients failed observation. Disequilibrium and larger tumor size were seen more often in the “failure group.” Authors conclude that VS patients presenting with disequilibrium and larger tumor size (14 vs 8.4 mm) should be followed more closely.
|
|
Stangerup et al, 2008
|
Retrospective study of 636 unilateral VS patients managed conservatively with annual MRI and audiogram for 10 years.
|
III
|
At diagnosis, 53% had good hearing and speech discrimination >70%. After 10 years observation, 31% met above criteria. At diagnosis: 17% had speech discrimination of 100%. After 10 years observation: 88% still had good hearing. Authors conclude that in patients with small tumors and normal speech discrimination the main indication for treatment should be tumor growth.
|
|
Ferri et al, 2008
|
Retrospective study of 123 unilateral VS patients followed prospectively with conservative treatment. Mean follow-up was 4.8 years; mean tumor size at diagnosis 11 mm; follow-up MRI every 6–12 months.
|
III
|
No growth observed in 64.5% of patients. 73.2% had hearing preservation during the follow-up, independent of growth. Only 45% patients presented with useful hearing (class A and B). Conservative management of VSs is safe, and treatment outcome are not affected by delay.
|
|
Solares et al, 2008
|
Retrospective study of 110 unilateral VS patients managed conservatively with at least 2 serial MRI scans. Mean follow-up was 31.4 months.
|
III
|
Overall, at 5 years, 70.6% showed no growth and 81.3% required no intervention. Tumor regression noted in 10%. For patients with intracanalicular tumors, at 5 years, 89.8% showed no growth, compared to 73.9% and 45.2% for larger tumors. Generally, recommend observation as initial management, particularly in patients with small tumors.
|
|
Roche et al, 2008
|
Retrospective study of 47 unilateral VS patients managed conservatively with mean follow-up of 43.8 months.
|
III
|
74% of patients failed conservative management. Data suggest that wait and see policy exposes patients to tumor growth.
|
|
Jeyakumar et al, 2007
|
Retrospective study of 120 unilateral VS patients divided into 2 groups: incidental and symptomatic.
|
III
|
12% had incidental diagnosis. Speech discrimination score asymmetry greater in symptomatic group. Tumor size larger in symptomatic group 1.5 cm vs 1.09 cm. Patients in symptomatic group more likely to undergo treatment (76% vs 47%)
|
|
Herwadker et al, 2005
|
Retrospective study of 50 unilateral VS patients managed conservatively.
|
III
|
There was no relationship between tumor size at diagnosis, patient age, sex, or tumor laterality. Authors conclude that clinical features available at presentation have no power to predict the expected behavior of sporadic VSs.
|
|
Lin et al, 2005
|
Retrospective study of unilateral VS patients divided into three groups: SRS = 42; SRT = 113; observation = 86.
|
III
|
Hearing outcome with VS is poor, however worsened by treatment. Authors recommended observation.
|
|
Raut et al, 2004
|
Retrospective study of 72 unilateral VS patients managed conservatively; mean follow-up 80 months.
|
III
|
Mean tumor growth was 1 mm/year. Mean growth rate for CPA tumors > IAC tumors, 1.3 mm/year vs 0 mm/year. 32% failed conservative management. Hearing deterioration occurred irrespective of tumor growth. No factors predictive of tumor growth/failure of conservative management were found.
|
|
Shin et al, 2000
|
Retrospective study of 97 unilateral VS patients managed conservatively; mean follow-up 31 months.
|
III
|
Mean tumor growth rate was 1.52 mm/year. 38% failed conservative management. Growth patterns were variable and not constant: Unpredictable growth patterns with 5 types observed.
|
|
Thomsen et al, 2000
|
Retrospective study of 40 intracanalicular unilateral VS patients managed conservatively; mean follow-up 3.6 years.
|
III
|
67.5% revealed growth. Four growth patterns were observed. Difficult to predict need for treatment based on variable growth patterns.
|
|
Yamamoto et al, 1998
|
Retrospective study of 12 unilateral VS patients managed conservatively followed prospectively; mean follow-up 564 days (18.8 months)
|
III
|
62% demonstrated significant tumor growth or symptom progression and required treatment.
|
|
Deen et al, 1996
|
Retrospective study of 68 unilateral VS patients managed conservatively.
|
III
|
Observation is reasonable treatment with diligent MRI follow-up
|
|
Bederson et al, 1991
|
Retrospective study of 70 unilateral VS patients managed conservatively; mean follow-up 2 years.
|
III
|
40% showed no growth. Average growth was 1.6 ± 0.4 at year 1 and 1.9 ± 1.0 at year 2.
|
GK, Gamma Knife; IAC, internal acoustic canal; MRI, magnetic resonance imaging; PTA, pure tone average; SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy; VS, vestibular schwannoma; WRS, word recognition score.
Table 3A. Outcome using Gamma Knife
|
Author/Year
|
Study Design
|
Class of Evidence
|
Study Conclusions Specific to Questions
|
|
Boari et al, 2014
|
Retrospective study of 379 VS patients; mean follow-up 75.7 months; median tumor volume = 1.2 cm3; median margin dose = 13 Gy
|
III
|
Radiographic control rate was 97%. Overall hearing preservation rate was 49%, 71% for GR class I patients and 93% for GR class I patients, 55 years old. Facial nerve paralysis rate was 2.9% transient and 1.1% permanent. Trigeminal nerve paralysis rate was 6.9% and 1.8% permanent. New onset or worsening of vertigo was 7.9% (73% resolved). Tinnitus worsened in 4.7%. Hydrocephalus was noted in 5.3% and was symptomatic in 1.1%.
|
|
Bir et al, 2014
|
Retrospective study of 82 VS patients; mean follow-up 4.7 years; average tumor size = 3.24 cm3; maximum margin dose = 12-13 Gy.
|
III
|
Radiographic control rate was 90%. Hearing preservation rate was 90%, 83%, and 58% at 3, 5, 10 years. Facial palsy rate was 5%, trigeminal palsy 4%, hydrocephalus 1%. KPS significantly improved from 79 KPS before SRS to 90 post-SRS. SRS improves QOL in patients with VSs.
|
|
Llopez Carratala et al, 2014
|
Retrospective study of 35 VS patients; mean follow-up 4.7 years; median tumor diameter = 15.7 mm; mean margin dose = 12 Gy.
|
III
|
Radiographic control rate was 90%. Hearing preservation rate was 65.7% at 10 years. There was no permanent CN paralysis, 8% of the patients had a transient facial nerve paralysis.
|
|
Wangerid et al, 2014
|
Retrospective study of 128 VS patients; median follow-up 7 years; mean tumor volume = 1.65 cm3; mean dose = 12.5 Gy.
|
III
|
Radiographic control 92%. Facial palsy rate was 3%, trigeminal 2%, hydrocephalus 3% with patients requiring CSF shunt. SRS results in high tumor control and low morbidity
|
|
Lunsford et al, 2013
|
Retrospective study of 829 VS patients; median tumor volume = 2.5 cc; median dose = 13 Gy.
|
III
|
Radiographic control rate was 97% at 10 years. Hearing preservation rate was 50% to 77%. Facial nerve palsy rate was 1% and trigeminal was 3%.
|
|
Zeiler et al, 2013
|
Retrospective study of 28 VS patients; mean follow-up 34.5 months; mean tumor diameter 3–4 cm.
|
III
|
Radiographic control rate was 92%. Hearing preservation rate was 100%. There was no new permanent CN paralysis, hydrocephalus developed in 16% of patients.
|
|
Williams et al, 2013
|
Retrospective study of 24 VS patients with tumor volume >3 cm compared to 49 patients with tumor volume <3 cm; median follow-up was 6.8 years (large) and 9.3 years (small); median dose: 11 Gy (large) 12 Gy (small).
|
III
|
Actuarial PFS was 95.2% (3 years) and 81.8% (5 years) for large VS compared to 97% (3 years) and 90% (5 years) for small VSs. Overall clinical outcome was better for small VSs with facial palsy rate 30%, trigeminal palsy in 30% and hydrocephalus in 8% in large VSs. SRS in patients with large VSs associated with worse PFS and clinical outcome than in patients with smaller tumor; however, it is a reasonable option for selected patients.
|
|
Wowra et al, 2013
|
Retrospective study of 111 VS patients; median follow-up 8.6 years; mean tumor volume = 1.6 cm3.
|
III
|
Radiographic control 95% at 6 years. Facial palsy rate 0%; trigeminal 11.7%.
|
|
Yang et al, 2013
|
Retrospective study of 65 VS patients; median follow-up 36 months; tumor dimension 3–4 cm.
|
III
|
Radiographic control rate at 2 years was 89%; 3% required surgery within 6 months because of progressive symptoms. At 2 years, 82% retained serviceable hearing. Facial nerve palsy rate was 2%, trigeminal 6%, hydrocephalus requiring CSF shunt 5%. Univariate analysis factor that predicted less likelihood of tumor control: prior resection, tumor volume >10 cc.
|
|
Van Eck et al, 2013 and 2005
|
Retrospective study of 78 VS patients; mean follow-up = 22 months; mean tumor volume = 2.28 cc3; mean margin dose = 13 Gy.
|
III
|
Radiographic control rate was 87%. Hearing preservation rate was 83.4%. Facial palsy rate was 1%, trigeminal 2%.
|
|
Yomo et al, 2012
|
Retrospective study of 154 VS patients; mean margin dose = 12.1 Gy.
|
III
|
Radiographic control rate was 95%. Maximum cochlear dose <4 Gy was the sole prognostic factor for hearing preservation. There was a trend indicating reduction in hearing preservation after SRS compared to conservative management.
|
|
Varughese et al, 2012
|
Retrospective review of prospective follow up of 45 VS patients .
|
III
|
Radiographic control rate was 71%. Highest odds for tumor control are found in older patients with larger tumors.
|
|
Hasegawa et al, 2011
|
Retrospective review of prospective follow-up of 117 VS patients; median tumor volume = 1.9 cm3; median margin dose = 12 Gy; median follow-up 74 months.
|
III
|
Radiographic control rate was 97.5%. Actuarial hearing preservation rate was 55% at 3 years and 34% at 8 years. In a limited number of patients treated with most recent planning techniques and who were GR class I pre-SRS: 3-year hearing preservation was 80% and this decreased to 70% at 5 years. In order to retain serviceable hearing, authors recommend treating patients while still GR class I.
|
|
Gerosa et al, 2010
|
Retrospective review of 74 VS patients; median dose = 12.4 Gy; median follow-up 50 months.
|
III
|
Radiographic control rate was 96%. Hearing preservation rate was 72% and 81% in GR class I. Tinnitus decreased from 52% to 28%, vestibular function improved by approximately 30%.
|
|
Franzin et al, 2009
|
Retrospective review of 50 VS patients; median dose = 13 Gy; median follow-up 36 months.
|
III
|
Radiographic control rate was 96%. Overall hearing preservation rate was 68% and 100% in patients with intracanalicular tumors. Prognostic factors for hearing preservation included: GR class I; age <54 years; intracanalicular tumors; presenting symptoms other than hearing loss.
|
|
Lobato-Polo et al, 2009
|
Retrospective study of 55 VS patients; mean follow ≥4 years; median tumor volume = 1.7 mm; median dose = 13 Gy.
|
III
|
Overall radiographic control rate was 96%. Hearing preservation rate was 93%, 87%, and 87% at 3, 5, and 10 years. Overall, facial nerve palsy rate was 1.8% and trigeminal 3.6%. In patients treated with dose ≤13 Gy facial nerve palsy rate was 0% and trigeminal 0%.
|
|
Fukuoka et al, 2009
|
Retrospective review of 152 VS patients; median dose = 12 Gy; median follow-up 5 years; median tumor volume = 2.0 cm3.
|
III
|
Radiographic control rate was 94% at 5 years and 92.4% at 8 years. Hearing preservation rate was 71%. Facial palsy rate 0%, trigeminal 2%, transient dizziness 17%, persistent dizziness 2%, hydrocephalus 5.3%.
|
|
Pollock et al, 2009
|
Retrospective review of 293 VS patients; median dose = 13 Gy; median follow-up 24 months.
|
III
|
Radiographic control rate was 96% at 3 years and 94% at 7 years. Multivariate analysis showed positive relationship between decreased radiographic control and increased numbers of isocenters.
|
|
Bush et al, 2008
|
Retrospective review of 17 VS patients; median dose = 13.8 Gy; median follow-up 33.6 months.
|
III
|
Radiographic control rate was 100%. Significant decrease in pure tone audiogram and word recognition comparing before and after SRS
|
|
Chopra et al, 2007
|
Retrospective review of 216 VS patients; median dose = 12-13 Gy; median tumor size 1.3 cm3.
|
III
|
Radiographic control rate was 91 ± 3% at 10 years, hearing preservation rate was 44 ± 12%, defined as no change from pre-SRS. Facial nerve palsy rate was zero.
|
|
Iwai et al, 2008
|
Retrospective review of 25 intracanalicular VS patients; median dose = 12 Gy; median tumor volume = 0.27 cm3; mean follow-up = 89 months.
|
III
|
Radiographic control rate was 96% at 10 years, hearing preservation rate was 64%. Hearing deterioration occurred 12-24 months post-SRS. Cranial nerve palsy rate was zero.
|
|
Kim et al, 2007
|
Retrospective review of 59 VS patients; dose = 11-13 Gy; follow-up = 5-year minimum.
|
III
|
Radiographic control rate was 97%, transient increase in size was found in 29% of cases. Hearing preservation rate was 33%. Hearing deterioration occurred 12–24 months post-SRS. Cranial nerve palsy rate was zero.
|
|
Liu et al, 2006
|
Retrospective study of 74 VS patients; mean follow-up 68.3 months; median tumor volume = 10 ± 5 cc; dose = 10–14 Gy.
|
III
|
Overall radiographic control rate was 96%. Facial nerve palsy rate was 4% and trigeminal 7%.
|
|
Hasegawa et al, 2005
|
Retrospective review of 73 VS patients; dose = 14.6 Gy; median tumor volume = 6.3 cm3.
|
III
|
Overall radiographic control rate was 87% at 10 years and 93% in patients with tumor volume <10 cm3. Hearing preservation rate was 37%. Facial nerve palsy rate was 11% and trigeminal 8%.
|
|
Huang et al, 2005
|
Retrospective review of 45 VS patients; dose = 11.5 Gy; median follow-up = 25 months; mean volume = 4.5 cc.
|
III
|
Overall radiographic control rate was 95.6%. Hearing preservation rate was 28.9%. Facial nerve palsy rate was zero, trigeminal 2%.
|
|
Inoue et al, 2013
|
Retrospective review of 18 VS patients .
|
III
|
Overall radiographic control rate was 93%. Hearing preservation rate was 80%. Facial nerve palsy rate was 0% and trigeminal 0%.
|
|
Flickinger et al, 2004 and 2000
|
Retrospective review of 313 VS patients; dose = 12–13 Gy; follow-up = 24 months; median tumor volume = 1.1 cc3.
|
III
|
Overall radiographic control rate was 98.6 ± 1.1%. Hearing preservation rate was 78.6 ± 5%. Facial nerve palsy rate was zero. Trigeminal function was preserved in 95.6 ± 5.8%.
|
|
Landy et al, 2004
|
Retrospective study of 34 VS patients; follow-up ≥1 year; dose = 10–14 Gy.
|
III
|
Overall radiographic control rate was 97%. Facial nerve palsy rate was 0%.
|
|
Iwai et al, 2003
|
Retrospective study of 25 VS patients; mean follow-up = 89 months; median tumor volume = 0.27 cm3; median dose = 12 Gy.
|
III
|
Overall radiographic control rate was 96%. Hearing preservation rate was 64%.
|
|
Unger et al, 2002
|
Retrospective review of 278 VS patients; median dose = 12 Gy; median follow-up = 88 months; median tumor volume = 3.8 cm3.
|
III
|
Overall radiographic control rate was 93% at 7 years. Facial nerve palsy rate was 8% and trigeminal 5%.
|
|
Kwon et al, 1999
|
Retrospective study of 102 VS patients; mean follow-up 55 months.
|
III
|
Overall radiographic control rate was 91%. with transient increase in size in 6%.
|
|
Vermeulen et al, 1998
|
Retrospective review of 14 intracanalicular VS patients; mean dose = 16 Gy; median follow-up = 18 months; mean tumor volume < 1 cm3.
|
III
|
Overall radiographic control rate was 100%. Facial nerve palsy rate was 43%, trigeminal 21%, balance disorder 14%, dizziness 7%, headache 7%.
|
|
Kondziolka et al, 1998
|
Retrospective review of 162 VS patients; mean dose = 16 Gy; median follow-up = 18 months; mean transverse diameter = 22 mm
|
III
|
Radiographic control rate was 98%. Hearing preservation 51%. Facial nerve palsy rate was 21%, trigeminal 27%. Any new or worsened deficit occurred within 28 months of treatment. Complete facial weakness only seen in patients with pre-existing deficit, usually after previous resection.
|
CN, cranial nerve; CSF, cerebrospinal fluid; GR, Gardner–Roberts; KPS, Karnofsky performance scale; PFS, progression-free survival; QOL, quality of life; SRS, stereotactic radiosurgery; VS, vestibular schwannoma.
Table 3B. Outcome using linear acceleration
|
Author/Year
|
Study Description
|
Class of Evidence
|
Study Conclusions Specific to Questions
|
|
Benghiat et al, 2014
|
Retrospective review of 97 VS patients; dose = 12 Gy; median follow-up = 2.4 years.
|
III
|
Overall radiographic control rate was 100%. Permanent facial nerve palsy rate was 2% and trigeminal 8%.
|
|
Lo et al, 2014
|
Retrospective review of 26 VS patients; mean dose = 11.8 ± 1.7 Gy; average follow-up = 57 months; mean tumor size = 19.7 ± 7.2mm.
|
III
|
Overall radiographic control rate was 88.5% at 6 years. Hearing preservation rate was 87%. Facial nerve palsy rate was 0% and trigeminal 0%.
|
|
Badakhshi et al, 2014
|
Retrospective review of 190 VS patients; dose = 13.5 Gy; median follow-up = 40 months.
|
III
|
Radiographic control rate was 88%. Hearing worsened in 27% of patients. Facial palsy rate was 1.1% and trigeminal 21.6%, dizziness 14.3%, tinnitus 12.6%
|
|
Combs et al, 2013
|
Retrospective review of 32 VS patients; median dose = 13 Gy; mean tumor volume = 1.2 cc.
|
III
|
Overall radiographic control rate was 93% at 10 years. Hearing preservation rate was 89.7%. Facial nerve palsy rate was 1% and trigeminal 2.1%
|
|
Roos et al, 2012
|
Retrospective review of 44 VS patients; mean dose = 12 Gy; mean transverse diameter = 21 mm.
|
III
|
Overall radiographic control rate was 97.7% and 97.1% for patients with 10-year median follow-up. Hearing preservation rate was 29%. Facial nerve palsy rate was 2% and trigeminal 11%.
|
|
Roos et al, 2011
|
Retrospective review of 84 VS patients; median dose = 12 Gy; median tumor diameter = 22 mm.
|
III
|
Overall radiographic control rate was 97.7 %. Hearing preservation rate was 38%. Estimated risk for hearing loss post-SRS for patients with initial PTA = 20 dB was 5 times greater than with PTA <20 dB. The authors noted a steady hearing decline out to at least 10 years.
|
|
Friedman et al, 2006
|
Retrospective review of 390 VS patients; median follow up = 40 months median dose = 12.5 Gy PTV = 22 mm3.
|
III
|
Overall radiographic control rate was 90% at 5 years. Facial nerve palsy rate was 4.4% (0.7% for dose <12.5 Gy) and trigeminal 3.6% (0.7% for dose <12.5 Gy).
|
|
Rutten et al, 2007
|
Retrospective review of 26 VS patients; mean dose = 13 Gy; median follow up = 110 months; mean tumor diameter = 15 mm.
|
III
|
Actuarial radiographic control probability was 91%. Hearing preservation rate was 55% at 9 years. Facial nerve palsy rate was 5% and trigeminal 8%.
|
|
Spiegelmann et al, 2001
|
Retrospective review of 44 VS patients; mean dose = 14.5 Gy; median follow up = 32 months; maximum diameter = 30 mm.
|
III
|
Overall radiographic control rate was 98%. Hearing preservation rate was 71% at 2.6 years. Facial nerve palsy rate was 8% and trigeminal 18%. The incidence of cranial neuropathy correlated with higher doses, particularly in large tumors >4 cm.
|
|
Suh et al, 2006
|
Retrospective review of 29 VS patients; mean dose = 16 Gy; median follow up = 49 months; median tumor volume = 21 mm3.
|
III
|
Overall radiographic control rate was 94% at 5 years. Hearing preservation rate was 36%. Facial nerve palsy rate was 32% and trigeminal 15%. In conclusion, a high prescription dose results in high cranial nerve palsy rate.
|
|
Mendenhall et al, 1996
|
Retrospective review of 56 VS patients; dose range 10-22 Gy; minimum follow up = 12 months.
|
|
Overall radiographic control rate was 93% at 5 years. Complication rate was 23%; the likelihood of complications correlated with higher dose and greater tumor volume.
|
PTA, pure tone average; PTV, planning target volume; SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy; VS, vestibular schwannoma.
Table 3C. Outcome using proton beam
|
Author/Year
|
Study Design
|
Class of Evidence
|
Study Conclusions Specific to Questions
|
|
Vernimmen et al, 2009
|
Retrospective study of 51 VS patients treated with dose of 26 CGyE in 3 fractions.
|
III
|
Local control was 98% at 5 years with hearing preservation of 42%, facial nerve preservation of 90.5%, and trigeminal nerve preservation of 93%. Hypofractionation proton beam offers excellent radiographic control and outcome in VS patients.
|
|
Weber et al, 2003
|
Retrospective study of 88 VS patients treated with dose of 12 CGyE in a single fraction; median tumor volume 1.4 cm3; 17% had already undergone surgical resection.
|
III
|
Local control was 95.3% and 93.6% at 2 and 5 years respectively. Three patients (3.4%) developed hydrocephalus and required shunting. Of the 21 patients with baseline serviceable hearing, 33% retained serviceable hearing. Facial nerve and trigeminal nerve preservation at 5 years was 91% and 89.4%, respectively. Proton beam offers excellent radiographic control and outcome in VS patients.
|
|
Bush et al, 2002
|
Retrospective study of 39 VS patients with mean follow-up 34 months and tumor volume 4.3 cm. Dose 54 Gy for patients with usable hearing, 60 Gy for deaf patients in 30-33 fractions.
|
III
|
Radiographic control was obtained in 100%. Hearing preservation reported in 31%, no CN deficit. Fractionated proton beam provides excellent control for VS.
|
|
Harsh et al, 2002
|
Retrospective study of 68 VS patients with mean follow up 44 months and tumor volume 2.5 cm. Dose 12Gy
|
III
|
Radiographic control was obtained in 94% at 2 years; 84% at 5 years. Hearing preservation reported in 33%, facial nerve deficit 5%; trigeminal deficit 5%, hydrocephalus 5%. Proton beam provides good control for VS.
|
CGyE, cobalt gray equivalent; CN, cranial nerve; SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy; VS, vestibular schwannoma.
Table 4. Dose delivered and outcome in vestibular schwannoma patients treated with radiosurgery/radiation therapy
|
Author/Year
|
Study Design
|
Class of Evidence
|
Study Conclusions Specific to Question
|
|
Hasegawa et al, 2013
|
Retrospective study of 440 patients who underwent SRS. High and low margin doses were assessed.
|
III
|
At a dose of ≤13 Gy, tumor control was 91% at 5 years, hearing preservation was 37% at 5 years, and facial palsy was 1%. For doses of >13 Gy with SRS, tumor control was 96% at 5 years, hearing preservation was 19% at 5 years, and facial palsy was 4.9%.
|
|
Pollock et al, 2013
|
Retrospective review of 293 patients treated with SRS and followed for a mean of 60.9 months.
|
III
|
Tumor progression was associated with a tumor margin dose ≤13 Gy.
|
|
Prasad et al, 2013
|
Retrospective study of 153 patients treated with SRS.
|
III
|
Hearing preservation was 47% for those treated with >13 Gy and 76% for those treated with ≤13 Gy.
|
|
Andrews et al, 2009
|
Retrospective study of 89 patients treated with SRT. A group of 43 were treated with SRT using a dose of 50.4 Gy and followed for a median of 53 weeks. Another group of 46 were treated with a dose of 46.7 Gy and followed for a median of 65 weeks.
|
III
|
Progression-free survival was 100% in both groups. Hearing preservation was 68% in the high dose group and the group had 0% facial palsy. In the low dose group, hearing preservation was 79%, and there was 2.2% risk of facial palsy.
|
|
Hudgins et al, 2006
|
Retrospective review of 159 patients treated with SRS. Low dose (≤14 Gy) was compared to high dose (>14 Gy).
|
III
|
Those treated with low dose had a progression-free survival of 94.8%, whereas those treated with high dose were 97.7%. There was no statistically significant difference in tumor control between groups.
|
|
Williams et al, 2002
|
A retrospective study of 249 patients treated with SRT.
|
III
|
Progression-free survival was 100%. Hearing preservation was 100% in those treated with 10 × 3 Gy and 88% in those treated with 5 Gy × 5 at 2 years.
|
SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy.
Table 5. Outcome after single fraction stereotactic radiosurgery or other fractionation schemes in vestibular schwannoma patients
|
Author/Year
|
Study Description
|
Class of Evidence
|
Conclusion Specific to Questions
|
|
Anderson et al, 2014
|
Retrospective study of 104 consecutively treated tumors in 103 patients. Patients were treated with SRS, HSRT, or SRT.
|
III
|
Progression-free survival in the SRS, HSRT, and SRT cohorts was 97%, 90.5%, and 100%, respectively. Hearing preservation rates for SRS, HSRT, and SRT were 60%, 63.2%, and 44.4%, respectively.
|
|
Badakhshi et al, 2014
|
Retrospective study of 190 patients with tumors <2 cm treated with SRS and 60 patients with tumors >2 cm to 3.5 cm treated with SRT.
|
III
|
Progression-free survival for SRS was 88%. Hearing preservation was not reported. Progression-free survival for SRT was 92%. Hearing preservation was not reported.
|
|
Puataweepong et al, 2014
|
Retrospective study of 39 tumors treated with SRS, 79 treated with hypofractionated SRS, and 28 treated with conventional SRT. Median follow-up was 61 months.
|
III
|
Progression-free survival for SRS was 95%, and hearing preservation was 75% at last follow-up.
Progression-free survival for HSRT was 100%, and hearing preservation was 87% at last follow-up.
Progression-free survival for SRT was 95%, and hearing preservation was 63% at last follow-up.
|
|
Combs et al, 2013
|
Retrospective follow-up of 248 tumors treated with either SRT or SRS.
|
III
|
Progression-free survival was overall 93%, and hearing preservation was overall 68.6% at 10 years.
|
|
Collen et al, 2011
|
Retrospective study of 78 patients treated with SRS and 41 treated with SRT. Median follow-up was 62 months.
|
III
|
Progression-free survival was overall 95%, and hearing preservation was 59% for SRS and 82% for SRT.
|
|
Kopp et al, 2011
|
Retrospective study of 115 patients treated with SRT or LINAC SRS. Patients were followed for a mean of 32.1 months in the SRT and 30.1 months in the SRS groups.
|
III
|
Progression-free survival for SRS was 98.5%, and hearing preservation was 85% at last follow-up.
|
|
Henzel et al, 2009
|
35 patients treated with SRS and 39 with SRT. Patients were followed for a minimum of 12 months.
|
III
|
Progression-free survival for SRS was 88.1% at 5 years, and hearing preservation was not reported. Progression-free survival for SRT was 87.5% at 5 years, and hearing preservation was not reported.
|
|
Meijer et al, 2003
|
Retrospective study of 129 patients treated with LINAC based SRS or SRT and followed for a mean of 33 months.
|
III
|
Progression-free survival for SRS was 100%, and hearing preservation was 75% at last follow-up.
Progression-free survival for SRT was 94%, and hearing preservation was 61% at last follow up.
|
|
Andrews et al, 2001
|
Retrospective study of 69 patients treated with Gamma Knife and 56 patients treated with LINAC SRT.
|
III
|
Progression-free survival was >97%. Hearing preservation was not reported.
|
SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy; HSRT, hypofractionated stereotactic radiotherapy; LINAC, linear acceleration.
Table 6. Follow-up imaging after radiosurgery/radiation treatment in patients with vestibular schwannoma
|
Author/Year
|
Study Description
|
Class of Evidence
|
Conclusions Specific to Questions
|
|
Matsuo et al, 2015
|
Retrospective review of 44 patients LINAC treatment where volume changes observed. MRI done every 3–4 months first 2 years; 6–12 months thereafter.
|
III
|
True enlargements should be considered increased volumes >2-fold and continued growth for at least 2 years.
|
|
Mindermann et al, 2013
|
Retrospective review of 225 patients GK; MRI 6 months, 1, 2, 3, 4, 5, and 2-year intervals thereafter.
|
III
|
Tumor progression occurs at 3–4 years; transient tumor expansion at about 6–18 months.
|
|
Nagano et al, 2010
|
Retrospective review of 87 patients GK; MRI every 3 months ×4; then every 6 months.
|
III
|
Peak tumor expansion 8.6 months; expansion average 68% of tumor volume. Careful serial follow-up MRI necessary for patients who harbor tumors with homogeneous enhancement.
|
|
Meijer et al, 2008
|
Retrospective review of 142 patients LINAC assessed with MRI at least 3 times over 32 months.
|
III
|
The first MRI at 2 years and the second at 5 years after SRS differentiated transient progression from ongoing progression.
|
|
Delsanti et al, 2008
|
Retrospective review of 322 patients GK; 3 MRIs after SRS.
|
III
|
Sequential MRIs are necessary. Significant increase noted in 178/332 at 6 months (54%). This was persisted albeit stable in 74/178 (42%) on follow-up MRIs.
|
|
Pollock et al, 2006
|
Retrospective review of 208 patients GK; MRI 6, 12, 24, and 48 months then biannually after radiosurgery.
|
III
|
Median time to tumor enlargement 9 months; median volume increase 75%. Only 2% showed progressive enlargement on serial images.
|
|
Okunaga et al, 2005
|
Retrospective review of 39 patients GK with MRI every 3–4 months.
|
III
|
Volumes changes beyond twofold or continuous enlargement for >2 years are key criteria in rating the effects of radiation.
|
|
Meijer et al, 2003
|
Retrospective review of 129 patients LINAC treatment single fraction vs fractionated. Patient followed with yearly MRI.
|
III
|
Follow-up imaging should include ventricles.
|
GK, Gamma Knife; MRI, magnetic resonance imaging; SRS, stereotactic radiosurgery; HSRT, hypofractionated stereotactic radiotherapy; LINAC, linear acceleration.
Table 7. Retreatment in vestibular schwannoma patients after treatment with radiosurgery/radiation therapy
|
Author/Year
|
Study Design
|
Class of Evidence
|
Study Conclusions Specific to Questions
|
|
Kano et al, 2010
|
Retrospective review of 6 patients at a single institution who had imaging evidence of tumor progression after initial SRS. All patients underwent retreatment with SRS. Median volume at initial SRS was 0.55 cc and 2.1 cc at second SRS. Initial treatment of median marginal dose of 13 Gy and 11 Gy on second treatment. Median time between initial and second SRS was 63 months. Median follow up was 29 months.
|
III
|
Tumor control (2 patients) and regression (4 patients) was achieved in all 6 patients. No patients developed significant adverse radiation effects or new neurological symptoms after the second SRS. This paper provides class III retrospective data that SRS can be used for tumor control after progression from init1al SRS treatment.
|
|
Liscak et al, 2009
|
Retrospective review of 26 patients retreated with SRS. 24 patients had follow up for a median of 43 months. Patients were treated with a median of 13 Gy to a median isodose of 50%.
|
III
|
15/24 tumors showed regression, 7/24 tumors were unchanged in size, and 2/24 tumors showed progression. 1/24 patients had deterioration of hearing and 4/24 developed facial symptoms (1 weakness, 3 facial spasm). 2/24 patients had preserved hearing prior to the retreatment and neither patient lost hearing after the second treatment. 1/19 patients with previously satisfactory facial nerve function experienced worsened facial function. 1/24 patients experienced vertigo after second GKS. 2/24 patients experience trigeminal neuropathy after second GKS. This paper provides class III retrospective data showing GKS can safely repeated when a vestibular schwannoma continues to grow despite previous GKS.
|
|
Dewan et al, 2008
|
Retrospective review of 11 patients at a single institution with unilateral VS retreated with GKS as a second radiation therapy after continued growth from a previous therapy. 10 patients were previously treated with GKS and one patient was previously treated with proton beam therapy. Patients received two treatments of 12 Gy. Mean time between treatments 51 months. Patients were evaluated at 6 months, 12 months, and annually for the first 5 years post treatment with MRI, audiological evaluation, and clinical examination.
|
III
|
2/11 VS showed increased size, 1/11 VS were unchanged, and 8/11 VS showed decreased size after retreatment with SRS. 2/11 patients experienced increased facial numbness, and 8/11 were unchanged or had improved facial numbness (1/11). There was no change in HB score after 2 treatments in any of the patients. 10/11 had non-functional hearing prior to the retreatment and 1/11 patients had decreased hearing after retreatment. 1/11 patients developed symptomatic radiation induced edema resulting in headaches and vertigo. This paper provides class III retrospective data that retreatment for GKS can be formed safely and effectively.
|
GKS, Gamma Knife surgery; MRI, magnetic resonance imaging; SRS, stereotactic radiosurgery; VS, vestibular schwannoma.
Table 8. Malignant transformation or tumorigenesis in vestibular schwannoma patients after radiosurgery/radiotherapy
|
Author/Year
|
Study Design
|
Class of Evidence
|
Study Conclusions Specific to Questions
|
|
Hasegawa et al, 2013
|
Retrospective review of 440 patients treated with GK surgery at a single institution. 347 patients underwent GK as initial treatment; 13 patients had NF2. Median follow-up of 12.5 years
|
III
|
1/440 patients developed malignant transformation (radiographic and histologic) representing an incidence of 0.3%. Annual incidence of malignant transformation was 0.02%. This paper provides class III retrospective data of the minimal risk of malignant transformation of VSs after GK treatment.
|
|
Rowe et al, 2007
|
Retrospective cohort study of patients with NF2 and von Hippel–Lindau treated with radiosurgery. 146 VSs were identified in 118 patients with NF2.
|
III
|
2 cases of malignant transformation (radiographic and histologic) were identified in 173 tumors in the NF2 population with radiosurgery. One case was identified as “malignant transformation” and the second case was identified as a glioblastoma. This paper provides class III retrospective data of the minimal, if any risk at all, of malignant transformation of VSs after SRS.
|
GK, Gamma Knife; NF2, neurofibromatosis type 2; SRS, stereotactic radiosurgery; VS, vestibular schwannoma.
Table 9. Indication for radiosurgery/radiation therapy in vestibular schwannoma patients with neurofibromatosis type 2
|
Author/Year
|
Study Design
|
Class of Evidence
|
Study Conclusions Specific to Questions
|
|
Choi et al, 2014
|
Retrospective single institution series analyzing clinical course of 25 pediatric NF2 patients. Median follow-up: 36 months.
|
III
|
Tumor control after SRS was 35.3% after 3 years. Hearing preservation after SRS was 67% at 1 year and 53% at 5 years. Treatment outcome for VS in children with NF2 was not favorable compared to previous reports of adults with NF2. Asymptomatic patients with VS who have ipsilateral normal hearing could be observed regardless of mass size even though it may be growing. Only when hearing deterioration occurs or other symptoms then treatment should be performed.
|
|
Sun et al, 2014
|
Retrospective single institution series of 73 VSs in 46 patients with NF2. Margin dose of 12.9 Gy. Median follow-up: 109 months; mean tumor size: 5.1 cm3.
|
III
|
Tumor control of 84%. Serviceable hearing after SRS was 31.9%. HN preservation at 3, 5, 10, and 15 years was 98%, 93%, 44%, and 17%. Of the 46 patients, 48% became deaf bilateral, 37% retained unilateral hearing, and 15% retained bilateral serviceable hearing. SRS provides long-term tumor control albeit less than in sporadic VSs. Treatment is dictated by tumor progression, size, and serviceable hearing. If one tumor is growing or the patient is developing hearing deterioration on one side, then that side should be treated. Rate of VS growth in NF2 patients decreased with increasing age.
|
|
Wagner et al, 2014
|
Retrospective review of 2 NF2 patients with VS treated with SRS. Local control rate of 94% for a follow-up of 131 months. Hearing preservation of 44%.
|
III
|
Local control rate of 94% for a follow-up of 131 months. Hearing preservation of 44%. In 2 patients, good long-term tumor control and hearing preservation.
|
|
Mallory et al, 2013
|
Prospective single institution series analyzing SRS in 26 NF2 patients with 27 VS tumors. 14 Gy at margin; median follow-up: 7. 6 years; median tumor size: 2.7 cm3.
|
III
|
84% tumors showed no growth. SRS is less effective than in sporadic VS. Higher margin doses achieved high tumor control but hearing preservation was lower. SRS may permit better use of cochlear implantation.
|
|
Sharma et al, 2010
|
Retrospective single institution series in 54 VSs of 30 NF patients. Median 12 Gy dose given. Median follow-up: 26.6 months.
|
III
|
Tumor control was 87.5% and hearing preservation 66.7% of cases. One patient with worsening FN function. SRS provides high tumor control and hearing preservation.
|
|
Phi et al, 2009
|
Retrospective single institution series analyzing 36 VSs in 30 NF patients. Margin dose of 12.1 Gy.
Clinical follow-up was 48.5 months and 36.5 months for radiographic. Mean tumor size was 3.2 cm3
|
III
|
Tumor control rates of 81, 74, and 66% in first, second, and fifth years. 5 tumors required surgery due to progression. Hearing preservation of 50%, 45%, and 33% in first, second, and fifth years. FN neuropathy reported in 1 patient.
|
|
Wentworth et al, 2009
|
Retrospective single institution series analyzing 20-year experience treating NF1 and NF2 patients undergoing RT for different tumors, including VS. 12/13 patients with VS underwent SRS; GK 12 Gy margin dose. After SRS, useful hearing in 3 VSs (2 patients)
|
III
|
Local control in 94% of patients. Useful hearing in 6/12 patients. Hearing preservation lower than in non-NF patients. However, 100% of patients will progress to bilateral deafness without treatment. 4/12 developed FN weakness (42%).
|
|
Meijer et al, 2008
|
Retrospective single institution series in 25 NF patients. 10–12.5 Gy. Mean follow-up was 51 months; mean tumor size: 2.5 cm.
|
III
|
10–12.5 Gy. Local tumor control was obtained in 100% of patients. No FN neuropathy. 40% retained hearing. Indication for SRS was tumor progression on MRI and/or progressive hearing loss. High tumor control rates.
|
|
Mathieu et al, 2007
|
Retrospective single institution series of 74 VS in 62 NF patients treated with GK SRS. Serviceable hearing in 35% of patients. Mean margin used was 14 and 27.5 Gy. Mean follow-up was 53 months.
|
III
|
Hearing preservation was 73% at 1 year, 59% at 2 years, and 48% at 5 years. FN weakness in 8% of patients. Tumor local control rates were 85%, 81%, and 81% at 5, 10, and 15 years. Results not as good as sporadic VS SRS, however, SRS should be strongly considered for primary management of VSs.
|
|
Vachhani et al, 2007
|
Retrospective single institution series of 14 VSs in 13 NF2 patients. Mean follow-up was 38 months.
|
III
|
100% local control at 1 year and 92% at 2 years and 5 years. In untreated contralateral tumors, local control was 100% at 1 year, 78% at 2 years, and 21% at 5 years. 78% maintained full FN function. SRS has a high local control rate for tumors in NF2 patients. No hearing preservation testing done.
|
|
Rowe et al, 2003
|
Retrospective single institution series of 122 NF2 in 96 patients treated with GK SRS. 20% of patients required surgery after SRS 8 years later. Margin dose was 13.4 Gy.
|
III
|
50% had local control of their tumor after 8 years. 40% retaining hearing after 3 years from SRS, 40% deterioration, and 20% deaf. FN neuropathy was 5%. SRS confers a significant advantage over natural history of VS in NF2 patients. Controls tumor growth and defers need for surgery.
|
|
Kida et al, 2000
|
Retrospective single institution series in 20 NF patients treated with SRS: 12 had profound ipsilateral hearing loss, 8 had serviceable hearing. Median follow-up: 33.6 months. Median tumor size: 24.4 mm.
|
III
|
Tumor control was 100%. Contralateral tumors were stable in 12 patients (60%) and enlarged in 8 (40%) patients. Preservation of hearing in 33.3%. FN neuropathy was 10%. Indications were growing tumor <30 mm in diameter, ipsilateral ear no serviceable hearing, and there is risk of brainstem compression
|
|
Subach et al, 1999
|
Retrospective single institution series in 40 NF patients. Mean follow-up was 36 months and mean tumor size 4.8 cc.
|
III
|
Overall tumor control was 98% at 36 months. Hearing preservation was 67%, and 81% had normal FN function. 10 patients with more than 5-year follow-up, 5 tumors smaller and 5 remained unchanged. Goal of SRS is arrest tumor growth while preserving neurological function. Safe and effective treatment. Better preservation of hearing. Patients with large tumors and progressive neurological deficits, due to brainstem compression, microsurgery is preferred. Tumor growth in NF2 patients should prompt SRS consideration.
|
|
Ito et al, 1997
|
Retrospective single institution series analyzing SRS treatment of VSs and complications. Margin doses of 12-25 Gy. NF2 patients at higher risk for hearing loss.
Number of patients: 46
Mean or median follow-up: 39 months
Mean or median tumor size: 12 mm
|
III
|
NF2 and tumor diameter were associated with hearing loss.
|
|
Linskey et al, 1992
|
Retrospective single institution series analyzing 17 NF2 patients after GK. Tumor margin dose was 14-20 Gy;
Mean follow-up: 1.4 years
|
III
|
Tumor control was 89.5%. Hearing preservation was 33%. Early study documenting tumor control after SRS when comparing to natural history of untreated contralateral VS in NF2 patients.
|
GK, Gamma Knife; FN, facial neuropathy; NF2, neurofibromatosis type 2; SRS, stereotactic radiosurgery; VS, vestibular schwannoma.
References
1. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 13 1951;102(4):316-319.
2. Germano IM. LINAC and GAMMA KNIFE RADIOSURGERY. Park Ridge, IL: American Association of Neurological Surgeons; 2000.
3. Gonzalez-Orus Alvarez-Morujo RJ, Alvarez-Palacios I, Martin-Oviedo C, Scola-Yurrita B, Aristegui-Ruiz MA. Conservative management of vestibular schwannoma. Acta Otorrinolaringol Esp 2014;65(5):275-282.
4. Breivik CN, Nilsen RM, Myrseth E, et al. Conservative management or gamma knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery 2013;73(1):48-56.
5. Ferri GG, Pirodda A, Ceroni AR, Fioravanti A, Calbucci F, Modugno GC. Management of growing vestibular schwannomas. Eur Arch Otorhinolaryngol 2013;270(7):2013-2019.
6. Regis J, Carron R, Park MC, et al. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas: clinical article. J Neurosurg 2013;119(suppl):105-111.
7. Pennings RJ, Morris DP, Clarke L, Allen S, Walling S, Bance ML. Natural history of hearing deterioration in intracanalicular vestibular schwannoma. Neurosurgery 2011;68(1):68-77.
8. Agrawal Y, Clark JH, Limb CJ, Niparko JK, Francis HW. Predictors of vestibular schwannoma growth and clinical implications. Otol Neurotol 2010;31(5):807-812.
9. Whitehouse K, Foroughi M, Shone G, Hatfield R. Vestibular schwannomas - when should conservative management be reconsidered? Br J Neurosurg 2010;24(2):185-190.
10. Bakkouri WE, Kania RE, Guichard JP, Lot G, Herman P, Huy PT. Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. J Neurosurg 2009;110(4):662-669.
11. Malhotra PS, Sharma P, Fishman MA, et al. Clinical, radiographic, and audiometric predictors in conservative management of vestibular schwannoma. Otol Neurotol 2009;30(4):507-514.
12. Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. Change in hearing during 'wait and scan' management of patients with vestibular schwannoma. J Laryngol Otol 2008;122(7):673-681.
13. Ferri GG, Modugno GC, Pirodda A, Fioravanti A, Calbucci F, Ceroni AR. Conservative management of vestibular schwannomas: an effective strategy. Laryngoscope 2008;118(6):951-957.
14. Solares CA, Panizza B. Vestibular schwannoma: an understanding of growth should influence management decisions. Otol Neurotol 2008;29(6):829-834.
15. Roche PH, Soumare O, Thomassin JM, Regis J. The wait and see strategy for intracanalicular vestibular schwannomas. Prog Neurol Surg 2008;21:83-88.
16. Jeyakumar A, Seth R, Brickman TM, Dutcher P. The prevalence and clinical course of patients with ‘incidental’ acoustic neuromas. Acta Otolaryngol 2007;127(10):1051-1057.
17. Herwadker A, Vokurka EA, Evans DG, Ramsden RT, Jackson A. Size and growth rate of sporadic vestibular schwannoma: predictive value of information available at presentation. Otol Neurotol 2005;26(1):86-92.
18. Lin VY, Stewart C, Grebenyuk J, et al. Unilateral acoustic neuromas: long-term hearing results in patients managed with fractionated stereotactic radiotherapy, hearing preservation surgery, and expectantly. Laryngoscope 2005;115(2):292-296.
19. Raut VV, Walsh RM, Bath AP, et al. Conservative management of vestibular schwannomas - second review of a prospective longitudinal study. Clin Otolaryngol Allied Sci 2004;29(5):505-514.
20. Shin YJ, Fraysse B, Cognard C, et al. Effectiveness of conservative management of acoustic neuromas. Am J Otol 2000;21(6):857-862.
21. Thomsen J, Charabi S, Tos M, Mantoni M, Charabi B. Intracanalicular vestibular schwannoma--therapeutic options. Acta Otolaryngol Suppl 2000;543:38-40.
22. Yamamoto M, Hagiwara S, Ide M, Jimbo M, Arai Y, Ono Y. Conservative management of acoustic neurinomas: prospective study of long-term changes in tumor volume and auditory function. Minim Invasive Neurosurg 1998;41(2):86-92.
23. Deen HG, Ebersold MJ, Harner SG, et al. Conservative management of acoustic neuroma: an outcome study. Neurosurgery 1996;39(2):260-264.
24. Bederson JB, von Ammon K, Wichmann WW, Yasargil MG. Conservative treatment of patients with acoustic tumors. Neurosurgery 1991;28(5):646-650.
25. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol 2013;15(suppl 2):ii1-ii56.
26. Van Gompel JJ, Patel J, Danner C, et al. Acoustic neuroma observation associated with an increase in symptomatic tinnitus: results of the 2007-2008 Acoustic Neuroma Association survey. J Neurosurg 2013;119(4):864-868.
27. Boari N, Bailo M, Gagliardi F, et al. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg 2014;121(suppl):123-142.
28. Bir SC, Ambekar S, Bollam P, Nanda A. Long-term outcome of gamma knife radiosurgery for vestibular schwannoma. J Neurol Surg B Skull Base 2014;75(4):273-278.
29. Llopez Carratala I, Escorihuela Garcia V, Orts Alborch M, de Paula Vernetta C, Marco Algarra J. Radiosurgery as treatment for acoustic neuroma. Ten years' experience. Acta Otorrinolaringol Esp 2014;65(6):327-331.
30. Wangerid T, Bartek Jr J, Svensson M, Forander P. Long-term quality of life and tumour control following gamma knife radiosurgery for vestibular schwannoma. Acta Neurochir (Wien) 2014;156(2):389-396.
31. Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg 2013;119(suppl):195-199.
32. Zeiler FA, Bigder M, Kaufmann A, et al. Gamma knife radiosurgery for large vestibular schwannomas: a Canadian experience. Can J Neurol Sci 2013;40(3):342-347.
33. Williams BJ, Xu Z, Salvetti DJ, McNeill IT, Larner J, Sheehan JP. Gamma Knife surgery for large vestibular schwannomas: a single-center retrospective case-matched comparison assessing the effect of lesion size. J Neurosurg 2013;119(2):463-471.
34. Wowra B, Muacevic A, Jess-Hempen A, Hempel JM, Muller-Schunk S, Tonn JC. Outpatient gamma knife surgery for vestibular schwannoma: definition of the therapeutic profile based on a 10-year experience. J Neurosurg 2013;119(suppl):114-118.
35. Yang HC, Kano H, Awan NR, et al. Gamma Knife radiosurgery for larger-volume vestibular schwannomas: clinical article. J Neurosurg 2013;119(suppl):801-807.
36. van Eck AT, Horstmann GA. Increased preservation of functional hearing after gamma knife surgery for vestibular schwannoma. J Neurosurg 2013;119(suppl):204-206.
37. Yomo S, Carron R, Thomassin JM, Roche PH, Regis J. Longitudinal analysis of hearing before and after radiosurgery for vestibular schwannoma. J Neurosurg 2012;117(5):877-885.
38. Varughese JK, Wentzel-Larsen T, Pedersen PH, Mahesparan R, Lund-Johansen M. Gamma knife treatment of growing vestibular schwannoma in Norway: a prospective study. Int J Radiat Oncol Biol Phys 1 2012;84(2):e161-e166.
39. Hasegawa T, Kida Y, Kato T, Iizuka H, Yamamoto T. Factors associated with hearing preservation after Gamma Knife surgery for vestibular schwannomas in patients who retain serviceable hearing. J Neurosurg 2011;115(6):1078-1086.
40. Gerosa M, Mesiano N, Longhi M, et al. Gamma Knife surgery in vestibular schwannomas: impact on the anterior and posterior labyrinth. J Neurosurg 2010;113(suppl):128-135.
41. Franzin A, Spatola G, Serra C, et al. Evaluation of hearing function after Gamma Knife surgery of vestibular schwannomas. Neurosurg Focus 2009;27(6):E3.
42. Lobato-Polo J, Kondziolka D, Zorro O, Kano H, Flickinger JC, Lunsford LD. Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery 2009;65(2):294-300.
43. Fukuoka S, Takanashi M, Hojyo A, Konishi M, Tanaka C, Nakamura H. Gamma knife radiosurgery for vestibular schwannomas. Prog Neurol Surg 2009;22:45-62.
44. Pollock BE, Link MJ, Foote RL. Failure rate of contemporary low-dose radiosurgical technique for vestibular schwannoma. J Neurosurg 2009;111(4):840-844.
45. Bush ML, Shinn JB, Young AB, Jones RO. Long-term hearing results in gamma knife radiosurgery for acoustic neuromas. Laryngoscope 2008;118(6):1019-1022.
46. Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2007;68(3):845-851.
47. Iwai Y, Yamanaka K, Kubo T, Aiba T. Gamma knife radiosurgery for intracanalicular acoustic neuromas. J Clin Neurosci 2008;15(9):993-997.
48. Kim KM, Park CK, Chung HT, Paek SH, Jung HW, Kim DG. Long-term outcomes of Gamma Knife stereotactic radiosurgery of vestibular schwannomas. J Korean Neurosurg Soc 2007;42(4):286-292.
49. Liu D, Xu D, Zhang Z, Zhang Y, Zheng L. Long-term outcomes after Gamma Knife surgery for vestibular schwannomas: a 10-year experience. J Neurosurg 2006;105(suppl):149-153.
50. Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg 2005;102(1):10-16.
51. Huang CF, Tu HT, Lo HK, Wang KL, Liu WS. Radiosurgery for vestibular schwannomas. J Chin Med Assoc 2005;68(7):315-320.
52. Inoue HK. Low-dose radiosurgery for large vestibular schwannomas: long-term results of functional preservation. J Neurosurg 2013;119(suppl):111-113.
53. Flickinger JC, Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford LD. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2004;60(1):225-230.
54. Landy HJ, Markoe AM, Wu X, et al. Safety and efficacy of tiered limited-dose gamma knife stereotactic radiosurgery for unilateral acoustic neuroma. Stereotact Funct Neurosurg 2004;82(4):147-152.
55. Iwai Y, Yamanaka K, Shiotani M, Uyama T. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery 2003;53(2):282-287.
56. Unger F, Walch C, Schrottner O, Eustacchio S, Sutter B, Pendl G. Cranial nerve preservation after radiosurgery of vestibular schwannomas. Acta Neurochir Suppl 2002;84:77-83.
57. Kwon Y, Khang SK, Kim CJ, Lee DJ, Lee JK, Kwun BD. Radiologic and histopathologic changes after Gamma Knife radiosurgery for acoustic schwannoma. Stereotact Funct Neurosurg 1999;72(suppl 1):2-10.
58. Vermeulen S, Young R, Posewitz A, et al. Stereotactic radiosurgery toxicity in the treatment of intracanalicular acoustic neuromas: the Seattle Northwest gamma knife experience. Stereotact Funct Neurosurg 1998;70 Suppl 1:80-87.
59. Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 1998;339(20):1426-1433.
60. Benghiat H, Heyes G, Nightingale P, et al. Linear accelerator stereotactic radiosurgery for vestibular schwannomas: a UK series. Clin Oncol (R Coll Radiol) 2014;26(6):309-315.
61. Lo WL, Yang KY, Huang YJ, Chen WF, Liao CC, Huang YH. Experience with Novalis stereotactic radiosurgery for vestibular schwannomas. Clin Neurol Neurosurg 2014;121:30-34.
62. Badakhshi H, Graf R, Bohmer D, Synowitz M, Wiener E, Budach V. Results for local control and functional outcome after linac-based image-guided stereotactic radiosurgery in 190 patients with vestibular schwannoma. J Radiat Res 2014;55(2):288-292.
63. Combs SE, Welzel T, Kessel K, et al. Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients' self-reported outcome. Radiother Oncol 2013;106(2):175-180.
64. Roos DE, Potter AE, Brophy BP. Stereotactic radiosurgery for acoustic neuromas: what happens long term? Int J Radiat Oncol Biol Phys 2012;82(4):1352-1355.
65. Roos DE, Potter AE, Zacest AC. Hearing preservation after low dose linac radiosurgery for acoustic neuroma depends on initial hearing and time. Radiother Oncol 2011;101(3):420-424.
66. Friedman WA, Bradshaw P, Myers A, Bova FJ. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg 2006;105(5):657-661.
67. Rutten I, Baumert BG, Seidel L, et al. Long-term follow-up reveals low toxicity of radiosurgery for vestibular schwannoma. Radiother Oncol 2007;82(1):83-89.
68. Spiegelmann R, Lidar Z, Gofman J, Alezra D, Hadani M, Pfeffer R. Linear accelerator radiosurgery for vestibular schwannoma. J Neurosurg 2001;94(1):7-13.
69. Suh JH, Barnett GH, Sohn JW, Kupelian PA, Cohen BH. Results of linear accelerator-based stereotactic radiosurgery for recurrent and newly diagnosed acoustic neuromas. Int J Cancer 20 2000;90(3):145-151.
70. Mendenhall WM, Friedman WA, Buatti JM, Bova FJ. Preliminary results of linear accelerator radiosurgery for acoustic schwannomas. J Neurosurg 1996;85(6):1013-1019.
71. Vernimmen FJ, Mohamed Z, Slabbert JP, Wilson J. Long-term results of stereotactic proton beam radiotherapy for acoustic neuromas. Radiother Oncol 2009;90(2):208-212.
72. Weber DC, Chan AW, Bussiere MR, et al. Proton beam radiosurgery for vestibular schwannoma: tumor control and cranial nerve toxicity. Neurosurgery 2003;53(3):577-586.
73. Bush DA, McAllister CJ, Loredo LN, Johnson WD, Slater JM, Slater JD. Fractionated proton beam radiotherapy for acoustic neuroma. Neurosurgery 2002;50(2):270-273.
74. Harsh GR, Thornton AF, Chapman PH, Bussiere MR, Rabinov JD, Loeffler JS. Proton beam stereotactic radiosurgery of vestibular schwannomas. Int J Radiat Oncol Biol Phys 2002;54(1):35-44.
75. Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg 2013;119(suppl):745-759.
76. Nakamura H, Jokura H, Takahashi K, Boku N, Akabane A, Yoshimoto T. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am J Neuroradiol 2000;21(8):1540-1546.
77. Nagano O, Serizawa T, Higuchi Y, et al. Tumor shrinkage of vestibular schwannomas after Gamma Knife surgery: results after more than 5 years of follow-up. J Neurosurg 2010;113(suppl):122-127.
78. Delsanti C, Roche PH, Thomassin JM, Regis J. Morphological changes of vestibular schwannomas after radiosurgical treatment: pitfalls and diagnosis of failure. Prog Neurol Surg 2008;21:93-97.
79. Yang HC, Kano H, Awan NR, et al. Gamma Knife radiosurgery for larger-volume vestibular schwannomas. Clinical article. J Neurosurg 2011;114(3):801-807.
80. Baschnagel AM, Chen PY, Bojrab D, et al. Hearing preservation in patients with vestibular schwannoma treated with Gamma Knife surgery. J Neurosurg 2013;118(3):571-578.
81. Han JH, Kim DG, Chung HT, et al. The risk factors of symptomatic communicating hydrocephalus after stereotactic radiosurgery for unilateral vestibular schwannoma: the implication of brain atrophy. Int J Radiat Oncol Biol Phys 2012;84(4):937-942.
82. Badakhshi H, Muellner S, Wiener E, Budach V. Image-guided stereotactic radiotherapy for patients with vestibular schwannoma. A clinical study. Strahlenther Onkol 2014;190(6):533-537.
83. Murphy ES, Barnett GH, Vogelbaum MA, et al. Long-term outcomes of Gamma Knife radiosurgery in patients with vestibular schwannomas. J Neurosurg 2011;114(2):432-440.
84. Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg 2013;118(3):557-565.
85. Pollock BE, Link MJ, Foote RL. Failure rate of contemporary low-dose radiosurgical technique for vestibular schwannoma. Clinical article. J Neurosurg 2013;119(suppl):840-844.
86. Andrews DW, Werner-Wasik M, Den RB, et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas: comparison of two dose cohorts. Int J Radiat Oncol Biol Phys 2009;74(2):419-426.
87. Hudgins WR, Antes KJ, Herbert MA, et al. Control of growth of vestibular schwannomas with low-dose Gamma Knife surgery. J Neurosurg 2006;105(suppl):154-160.
88. Williams JA. Fractionated stereotactic radiotherapy for acoustic neuromas. Int J Radiat Oncol Biol Phys 2002;54(2):500-504.
89. Puataweepong P, Dhanachai M, Dangprasert S, et al. Linac-based stereotactic radiosurgery and fractionated stereotactic radiotherapy for vestibular schwannomas: comparative observations of 139 patients treated at a single institution. J Radiat Res 2014;55(2):351-358.
90. Anderson BM, Khuntia D, Bentzen SM, et al. Single institution experience treating 104 vestibular schwannomas with fractionated stereotactic radiation therapy or stereotactic radiosurgery. J Neurooncol 2014;116(1):187-193.
91. Kopp C, Fauser C, Muller A, et al. Stereotactic fractionated radiotherapy and LINAC radiosurgery in the treatment of vestibular schwannoma-report about both stereotactic methods from a single institution. Int J Radiat Oncol Biol Phys 2011;80(5):1485-1491.
92. Collen C, Ampe B, Gevaert T, et al. Single fraction versus fractionated linac-based stereotactic radiotherapy for vestibular schwannoma: a single-institution experience. Int J Radiat Oncol Biol Phys 15 2011;81(4):e503-e509.
93. Henzel M, Hamm K, Sitter H, et al. Comparison of stereotactic radiosurgery and fractionated stereotactic radiotherapy of acoustic neurinomas according to 3-D tumor volume shrinkage and quality of life. Strahlenther Onkol 2009;185(9):567-573.
94. Meijer OW, Vandertop WP, Baayen JC, Slotman BJ. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study. Int J Radiat Oncol Biol Phys 2003;56(5):1390-1396.
95. Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys 2001;50(5):1265-1278.
96. Hansasuta A, Choi CY, Gibbs IC, et al. Multisession stereotactic radiosurgery for vestibular schwannomas: single-institution experience with 383 cases. Neurosurgery 2011;69(6):1200-1209.
97. Meijer OW, Wolbers JG, Baayen JC, Slotman BJ. Fractionated stereotactic radiation therapy and single high-dose radiosurgery for acoustic neuroma: early results of a prospective clinical study. Int J Radiat Oncol Biol Phys 2000;46(1):45-49.
98. Massager N, Nissim O, Delbrouck C, et al. Irradiation of cochlear structures during vestibular schwannoma radiosurgery and associated hearing outcome. J Neurosurg 2007;107(4):733-739.
99. Timmer FC, Hanssens PE, van Haren AE, et al. Gamma knife radiosurgery for vestibular schwannomas: results of hearing preservation in relation to the cochlear radiation dose. Laryngoscope 2009;119(6):1076-1081.
100. Matsuo T, Okunaga T, Kamada K, Izumo T, Hayashi N, Nagata I. Long-term follow-up results of linear accelerator-based radiosurgery for vestibular schwannoma using serial three-dimensional spoiled gradient-echo MRI. J Clin Neurosci 2015;22(2):320-325.
101. Mindermann T, Schlegel I. Grading of vestibular schwannomas and corresponding tumor volumes: ramifications for radiosurgery. Acta Neurochir (Wien) 2013;155(1):71-74.
102. Meijer OW, Weijmans EJ, Knol DL, et al. Tumor-volume changes after radiosurgery for vestibular schwannoma: implications for follow-up MR imaging protocol. AJNR Am J Neuroradiol 2008;29(5):906-910.
103. Pollock BE. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience. Neurosurgery 2006;58(2):241-248.
104. Okunaga T, Matsuo T, Hayashi N, et al. Linear accelerator radiosurgery for vestibular schwannoma: measuring tumor volume changes on serial three-dimensional spoiled gradient-echo magnetic resonance images. J Neurosurg 2005;103(1):53-58.
105. Kano H, Kondziolka D, Niranjan A, Flannery TJ, Flickinger JC, Lunsford LD. Repeat stereotactic radiosurgery for acoustic neuromas. Int J Radiat Oncol Biol Phys 2010;76(2):520-527.
106. Liscak R, Vladyka V, Urgosik D, Simonova G, Vymazal J. Repeated treatment of vestibular schwannomas after gamma knife radiosurgery. Acta Neurochir (Wien) 2009;151(4):317-324.
107. Dewan S, Noren G. Retreatment of vestibular schwannomas with Gamma Knife surgery. J Neurosurg 2008;109(suppl):144-148.
108. Rowe J, Grainger A, Walton L, Radatz M, Kemeny A. Safety of radiosurgery applied to conditions with abnormal tumor suppressor genes. Neurosurgery 2007;60(5):860-864.
109. Hayhurst C, Monsalves E, Bernstein M, et al. Predicting nonauditory adverse radiation effects following radiosurgery for vestibular schwannoma: a volume and dosimetric analysis. Int J Radiat Oncol Biol Phys 2012;82(5):2041-2046.
110. Linskey ME, Lunsford LD, Flickinger JC, Kondziolka D. Stereotactic radiosurgery for acoustic tumors. Neurosurg Clin N Am 1992;3(1):191-205.
111. van de Langenberg R, Dohmen AJ, de Bondt BJ, Nelemans PJ, Baumert BG, Stokroos RJ. Volume changes after stereotactic LINAC radiotherapy in vestibular schwannoma: control rate and growth patterns. Int J Radiat Oncol Biol Phys 2012;84(2):343-349.
112. Chuang CC, Chang CS, Tyan YS, Chuang KS, Tu HT, Huang CF. Use of apparent diffusion coefficients in evaluating the response of vestibular schwannomas to Gamma Knife surgery. J Neurosurg 2012;117(suppl):63-68.
113. Larjani S, Monsalves E, Pebdani H, et al. Identifying predictors of early growth response and adverse radiation effects of vestibular schwannomas to radiosurgery. PLoS One 2014;9(10):e110823.
114. Chung WY, Liu KD, Shiau CY, et al. Gamma knife surgery for vestibular schwannoma: 10-year experience of 195 cases. Journal of neurosurgery 2005;102(suppl):87-96.
115. Yu CP, Cheung JY, Leung S, Ho R. Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by gamma knife radiosurgery. J Neurosurg 2000;93(suppl 3):82-89.
116. Comey CH, McLaughlin MR, Jho HD, Martinez AJ, Lunsford LD. Death from a malignant cerebellopontine angle triton tumor despite stereotactic radiosurgery. Case report. J Neurosurg 1998;89(4):653-658.
117. Shamisa A, Bance M, Nag S, et al. Glioblastoma multiforme occurring in a patient treated with gamma knife surgery. Case report and review of the literature. J Neurosurg 2001;94(5):816-821.
118. Bari ME, Forster DM, Kemeny AA, Walton L, Hardy D, Anderson JR. Malignancy in a vestibular schwannoma. Report of a case with central neurofibromatosis, treated by both stereotactic radiosurgery and surgical excision, with a review of the literature. Br J Neurosurg 2002;16(3):284-289.
119. McEvoy AW, Kitchen ND. Rapid enlargement of a vestibular schwannoma following gamma knife treatment. Minim Invasive Neurosurg 2003;46(4):254-256.
120. Akamatsu Y, Murakami K, Watanabe M, Jokura H, Tominaga T. Malignant peripheral nerve sheath tumor arising from benign vestibular schwannoma treated by gamma knife radiosurgery after two previous surgeries: a case report with surgical and pathological observations. World Neurosurg 2010;73(6):751-754.
121. Husseini ST, Piccirillo E, Sanna M. On “malignant transformation of acoustic neuroma/vestibular schwannoma 10 years after gamma knife stereotactic radiosurgery” (skull base 2010;20:381-388). Skull Base 2011;21(2):135-138.
122. Schmitt WR, Carlson ML, Giannini C, Driscoll CL, Link MJ. Radiation-induced sarcoma in a large vestibular schwannoma following stereotactic radiosurgery: case report. Neurosurgery 2011;68(3):E840-846; discussion E846.
123. Milligan BD, Pollock BE, Foote RL, Link MJ. Long-term tumor control and cranial nerve outcomes following gamma knife surgery for larger-volume vestibular schwannomas. J Neurosurg 2012;116(3):598-604.
124. Lee CC, Yen YS, Pan DH, et al. Delayed microsurgery for vestibular schwannoma after gamma knife radiosurgery. J Neurooncol 2010;98(2):203-212.
125. Wagner J, Welzel T, Habermehl D, Debus J, Combs SE. Radiotherapy in patients with vestibular schwannoma and neurofibromatosis type 2: clinical results and review of the literature. Tumori 2014;100(2):189-194.
126. Sun S, Liu A. Long-term follow-up studies of Gamma Knife surgery for patients with neurofibromatosis type 2. J Neurosurg 2014;121(suppl):143-149.
127. Mallory GW, Pollock BE, Foote RL, Carlson ML, Driscoll CL, Link MJ. Stereotactic radiosurgery for neurofibromatosis 2-associated vestibular schwannomas: toward dose optimization for tumor control and functional outcomes. Neurosurgery 2014;74(3):292-300.
128. Choi JW, Lee JY, Phi JH, et al. Clinical course of vestibular schwannoma in pediatric neurofibromatosis type 2. J Neurosurg Pediatr 2014;13(6):650-657.
129. Sharma MS, Singh R, Kale SS, Agrawal D, Sharma BS, Mahapatra AK. Tumor control and hearing preservation after Gamma Knife radiosurgery for vestibular schwannomas in neurofibromatosis type 2. J Neurooncol 2010;98(2):265-270.
130. Wentworth S, Pinn M, Bourland JD, et al. Clinical experience with radiation therapy in the management of neurofibromatosis-associated central nervous system tumors. Int J Radiat Oncol Biol Phys 2009;73(1):208-213.
131. Phi JH, Kim DG, Chung HT, Lee J, Paek SH, Jung HW. Radiosurgical treatment of vestibular schwannomas in patients with neurofibromatosis type 2: tumor control and hearing preservation. Cancer 2009;115(2):390-398.
132. Meijer OW, Vandertop WP, Lagerwaard FJ, Slotman BJ. Linear accelerator-based stereotactic radiosurgery for bilateral vestibular schwannomas in patients with neurofibromatosis type 2. Neurosurgery 2008;62(5 suppl):A37-A42.
133. Vachhani JA, Friedman WA. Radiosurgery in patients with bilateral vestibular schwannomas. Stereotact Funct Neurosurg 2007;85(6):273-278.
134. Mathieu D, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery 2007;60(3):460-468.
135. Rowe JG, Radatz MW, Walton L, Soanes T, Rodgers J, Kemeny AA. Clinical experience with gamma knife stereotactic radiosurgery in the management of vestibular schwannomas secondary to type 2 neurofibromatosis. J Neurol Neurosurg Psychiatry 2003;74(9):1288-1293.
136. Kida Y, Kobayashi T, Tanaka T, Mori Y. Radiosurgery for bilateral neurinomas associated with neurofibromatosis type 2. Surg Neurol 2000;53(4):383-389.
137. Subach BR, Kondziolka D, Lunsford LD, Bissonette DJ, Flickinger JC, Maitz AH. Stereotactic radiosurgery in the management of acoustic neuromas associated with neurofibromatosis type 2. J Neurosurg 1999;90(5):815-822.
138. Ito K, Kurita H, Sugasawa K, Mizuno M, Sasaki T. Analyses of neuro-otological complications after radiosurgery for acoustic neurinomas. Int J Radiat Oncol Biol Phys 1997;39(5):983-988.
139. Linskey ME, Lunsford LD, Flickinger JC. Tumor control after stereotactic radiosurgery in neurofibromatosis patients with bilateral acoustic tumors. Neurosurgery 1992;31(5):829-838.
© Congress of Neurological Surgeons
Source: Neurosurgery, February 2018