Guidelines on the Management of Patients with Vestibular Schwannoma
4. Intraoperative Cranial Nerve Monitoring in Vestibular Schwannoma Surgery
download pdf Neurosurgery, 2017
Sponsored by: Congress of Neurological Surgeons (CNS) and the AANS/CNS Tumor Section
Endorsed by: Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)
Authors:
Esther X. Vivas, MD1, Matthew L. Carlson, MD2,3, Brian A. Neff, MD2,3, Neil T. Shepard, PhD2, D. Jay McCracken, MD4, Alex D. Sweeney, MD5, Jeffrey J. Olson, MD4
1 Department of Otolaryngology-Head & Neck Surgery, Emory University School of Medicine, Atlanta, Georgia, USA
2 Department of Otorhinolaryngology, Mayo Clinic, School of Medicine, Rochester, Minnesota, USA
3 Department of Neurosurgery, Mayo Clinic, School of Medicine, Rochester, Minnesota, USA
4Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
5Bobby R. Alford Department of Otolaryngology-Head and Neck Surgery, Baylor College of Medicine, Houston, Texas, USA
Correspondence:
Esther X. Vivas, M.D. Department of Otolaryngology-Head and Neck Surgery
Emory University
Medical Office Tower
550 Peachtree Street, Suite 1135<
Atlanta, Georgia 30308
Phone: 404-778-2178
Email: evivas@emory.edu
Keywords: ABR, acoustic neuroma, cranial nerve monitoring, electrophysiology, EMG, intraoperative cranial nerve monitoring, vestibular schwannoma
No part of this manuscript has been published or submitted for publication elsewhere.
Abbreviations
ABR: Auditory brainstem response
CMAP: Compound muscle action potential
DENM: Direct eighth cranial nerve monitoring
EMG: Electromyogram
FN: Facial nerve
GR: Gardner–Robertson facial function grading system
HB: House–Brackmann facial function grading system
ICNM: Intraoperative cranial nerve monitoring
NF: Neurofibromatosis
PPV: Positive predictive value
PTA: Pure tone average
SMS: Supramaximal stimulation
SRS: Speech recognition score
SRT: Speech reception threshold
VS: Vestibular schwannoma
WRS: Word recognition score
Abstract
Facial Nerve Monitoring
Question 1
Does intraoperative facial nerve monitoring during vestibular schwannoma surgery lead to better long-term facial nerve function?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery regardless of tumor characteristics.
Recommendation
Level 3: It is recommended that intraoperative facial nerve monitoring be routinely utilized during vestibular schwannoma surgery to improve long-term facial nerve function.
Question 2
Can intraoperative facial nerve monitoring be used to accurately predict favorable long-term facial nerve function after vestibular schwannoma surgery?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery.
Recommendation
Level 3: Intraoperative facial nerve monitoring can be used to accurately predict favorable long-term facial nerve function after vestibular schwannoma surgery. Specifically, the presence of favorable testing reliably portends a good long-term facial nerve outcome. However, the absence of favorable testing in the setting of an anatomically intact facial nerve does not reliably predict poor long-term function and therefore cannot be used to direct decision-making regarding need for early reinnervation procedures.
Question 3
Does an anatomically intact facial nerve with poor electromyogram electrical responses during intraoperative testing reliably predict poor long-term facial nerve function?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery.
Recommendation
Level 3: Poor intraoperative electromyogram electrical response of the facial nerve should not be used as a reliable predictor of poor long-term facial nerve function.
Cochlear Nerve Monitoring
Question 4
Should intraoperative eighth cranial nerve monitoring be used during vestibular schwannoma surgery?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery with measurable preoperative hearing levels and tumors smaller than 1.5 cm.
Recommendation
Level 3: Intraoperative eighth cranial nerve monitoring should be used during vestibular schwannoma surgery when hearing preservation is attempted.
Question 5
Is direct monitoring of the eighth cranial nerve superior to the use of far-field auditory brain stem responses?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery with measurable preoperative hearing levels and tumors smaller than 1.5 cm.
Recommendation
Level 3: There is insufficient evidence to make a definitive recommendation.
Introduction
Rationale
The surgical management of VSs has experienced a significant evolution since its inception by Harvey Cushing, MD, and other early pioneering surgeons of the late nineteenth and early twentieth centuries. In the 1960s, further progress was made with the implementation of the operating microscope and surgical drill, the use of which is largely credited to William House, MD. The coadvancement of surgical techniques and technology has led to a significant decline in the morbidity and mortality profile of VS surgery. Today, the neurologic deficits once considered acceptable sequelae are no longer commonplace, and mortality from surgery is reported at <1% when performed by experienced surgical teams.1
Early on, facial paralysis and deafness were thought to be inevitable and acceptable consequences of tumor resection, particularly because most patients were diagnosed with large and often life-threatening tumors. In today’s practice, however, the expectation is to preserve facial function in the vast majority of cases. As a result, patient quality of life following VS surgery has improved, and the sequelae of ophthalmologic complications and the need for invasive dynamic facial rehabilitation procedures have been reduced. A systematic review of the literature published in 2010 found an overall microsurgical FN preservation rate of 74%.2 The value placed in preserving seventh cranial nerve functional integrity is high and can motivate subtotal resection in select large tumors, with or without the use of postoperative radiation therapy.
Hearing preservation surgery is the latest chapter in the evolution of VS management. The advent and widespread availability of contrast-enhanced magnetic resonance imaging has allowed for earlier diagnosis, producing a population of patients with smaller tumors and better baseline hearing. Patients with small- to medium-sized tumors and serviceable hearing are now being offered hearing preservation surgery at higher rates than ever before. Whereas the translabyrinthine approach commits the patient to permanent ipsilateral deafness, the retrosigmoid and middle fossa approaches offer opportunities to preserve acoustic function in select tumors. Currently, postoperative FN function and hearing preservation are 2 primary benchmarks consistently reported by high-volume VS surgical centers. These 2 measures have been enhanced significantly by improvements in surgical technique and the development and refinement of intraoperative cranial nerve monitoring (ICNM).
Delgado et al3 introduced ICNM of the facial nerve in the late 1970s, which has become a mainstay for most VS surgeons.2 A consensus statement published by the National Institutes of Health in the 1990s recommended the routine use of neuromonitoring during VS surgery.4 The existing literature on this subject primarily consists of large retrospective case series from high-volume surgical centers – large prospective comparative studies are generally lacking. Furthermore, heterogeneous reporting and the use of inconsistent electroprognostic testing parameters are variable, rendering interstudy comparisons challenging. Recently these parameters have garnered a more elaborate role. Whereas the initial role of ICNM was for the identification and intraoperative mapping of the FN, there is a new focus on electrical factors that could potentially serve as electroprognostic indicators of long-term facial function. The utility of testing in this manner may have a profound impact on how to counsel patients with immediate postoperative paresis and an anatomically preserved FN. In addition, it would offer the treating physician an objective basis for proceeding with watchful waiting and conservative measures versus a recommendation that a patient undergo early dynamic facial reanimation procedures. An example would be timing VS surgery with hypoglossal-facial anastomosis (where an earlier intervention is associated with improved functional outcomes), as opposed to enrolling the patient into an observation period for spontaneous recovery that can last anywhere from 12 to 18 months.
In contrast to facial nerve monitoring, the role of ICNM for hearing preservation is less well defined and is not uniformly used. This may be the result of a more technically challenging and cumbersome process than what is required with FN monitoring. It may also have to do with differences in treatment philosophy for smaller tumors between surgeons and between centers.
There is currently a need to assess the existing literature for VS surgery outcomes, specifically as it relates to the use of ICNM and its impact on postoperative FN function and hearing preservation.
Objectives
The objective of this systematic review is to critically assess the existing literature and provide an evidence-based clinical practice guideline regarding the use of ICNM during VS surgery. Specifically, this systematic review focuses on intraoperative monitoring techniques and eletroprognostic parameters as they relate to posttreatment function of the seventh and eighth cranial nerves.
Methods
Process Overview
The evidence-based clinical practice guideline taskforce members and the Tumor Section of the American Association of Neurological Surgeons and the Congress of Neurological Surgeons (CNS) conducted a systematic review of the literature relevant to the management of VSs. The PubMed, Embase, and Web of Science databases were queried. The keywords used during our search of the medical literature databases cited above are documented in Tables 1 and 2. Additional details of the systematic review are provided below and within the introduction and methodology chapter of the guideline (here).
Article Inclusion/Exclusion Criteria
Citations were manually reviewed by the team with specific inclusion and exclusion criteria as outlined below. The duplicates from the search were eliminated. Two independent reviewers reviewed and abstracted full-text data for each article, and the 2 sets of data were compared for agreement by a third party. Inconsistencies were re-reviewed and disagreements were resolved by consensus. The evolution of the article selection is illustrated with flow diagrams (Figures 1 and 2). All citations that focused on adult patients and surgical treatment of VSs were broadly considered. For literature to be included for further consideration, papers had to meet the following criteria:
General
- Investigated patients suspected of having vestibular schwannomas
- Was of humans
- Was not an in vitro study
- Was not a biomechanical study
- Was not performed on cadavers
- Published between January 1, 1990 and December 31, 2014
- Published in a peer-reviewed journal
- Was not a meeting abstract, editorial, letter, or commentary
- Was published in English
- Included quantitatively presented results
Specific
- Used an established FN function grading system, such as the House–Brackmann (HB)5 scale or the Sunnybrook (SB)6 scale.
- Used the 1995 American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS)7 or Gardner–Robertson (GR)8 hearing classification system OR presented data using word recognition score (WRS) and pure tone average (PTA) for defining hearing status or had individual patient data presented such that the latter criteria could be applied and analyzed
- Included pre- and postoperative audiometric data
- Included a median or mean follow-up of 12 months following treatment when assessing long-term facial outcomes
- Included only studies evaluating intraoperative electrophysiological testing of the facial and cochlear nerves
- Used electrically evoked testing with EMG
- NF status was collected when available but was not an exclusion criterion
The authors did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents were developed using different inclusion criteria than those specified in our guideline. Therefore, they may have included studies that do not meet the inclusion criteria listed above. These documents were recalled if their abstract suggested that they might address one of the recommendations set forth in this guideline. The authors searched their bibliographies for additional studies.
Search Strategies
The task force collaborated with a medical librarian to search for articles published between January 1, 1990 and December 31, 2014. Three electronic databases were searched: PubMed, EMBASE, and Web of Science. Strategies for searching electronic databases were constructed by the evidence-based clinical practice guideline taskforce members and the medical librarian using previously published search strategies to identify relevant studies (Tables 1 and 2).
Searches of electronic databases were supplemented with manual screening of the bibliographies of all retrieved publications. Bibliographies of recent systematic reviews and other review articles for potentially relevant citations were also searched. All articles identified were subject to the study selection criteria listed above. The guideline committee also examined lists of included and excluded studies for errors and omissions. The guideline task force went to great lengths to obtain a complete set of relevant articles to ensure guideline recommendations are not based on a biased subset of articles. Two datasets were constructed, one for FN monitoring and another for cochlear nerve monitoring.
Facial Nerve Monitoring
The search of the 3 above mentioned databases yielded a total of 2853 candidate articles. One thousand nine hundred and eighty-four remained after duplicates were removed and date range criteria were applied. The abstracts were reviewed, and after the aforementioned general and specific inclusion/exclusion criteria were applied, 21 articles remained and were included in the final analysis (Table 1, Figure 1).
Cochlear Nerve Monitoring
The search of the 3 abovementioned databases yielded a total of 1849 articles. Eight hundred and three remained after duplicates were removed and date range criteria applied. The abstracts were reviewed, and after the aforementioned general and specific exclusion criteria were applied, 7 articles remained and were included in the final analysis (Table 2, Figure 2).
Data Analysis
Evidence tables for the use of intraoperative cochlear nerve monitoring and FN monitoring were constructed using key study parameters as outlined above.
Facial Nerve: Data extraction included study design, level of evidence, total number of patients, pre- and posttreatment facial function, study selection parameters, tumor characteristics, mean or median follow-up, neurofibromatosis type 2 status, and prognostic parameters associated with short- and long-term facial function.
Cochlear Nerve: Data extraction included study design, level of evidence, total number of patients, pre- and posttreatment hearing status, study selection parameters, tumor characteristics, mean or median follow-up, neurofibromatosis type 2 status, and prognostic features associated with postoperative hearing preservation.
Classification of Evidence and Guideline Formulation
The concept of linking evidence to recommendations has been further formalized by the American Medical Association (AMA) and many specialty societies, including the American Association of Neurological Surgeons (AANS), the CNS, and the American Academy of Neurology (AAN). This formalization involves the designation of specific relationships between the strength of evidence and the strength of recommendations to avoid ambiguity. In the paradigm for prognostication used in this guideline, evidence is classified into 1 of 3 tiers based on the degree at which the study fulfills the 5 technical criteria listed below:
- Was a well-defined representative sample of patients assembled at a common (usually early) point in the course of their disease?
- Was patient follow-up sufficiently long and complete?
- Were objective outcome criteria applied in a “blinded” fashion?
- If subgroups with different prognoses were identified, was there adjustment for important prognostic factors?
- If specific prognostic factors were identified, was there validation in an independent “test set” group of patients?
Class I evidence is used to support recommendations of the strongest type, defined as Level 1 recommendations, and require that all 5 technical criteria are satisfied. Class II evidence supports intermediate strength recommendations, defined as level 2 recommendations, and requires that 4 of the 5 technical criteria be met. Class III evidence supports Level 3 recommendations, comprising all remaining studies that satisfy ≤3 of the 5 technical criteria. A basis for these guidelines can be viewed in Haines SJ and Nicholas JS (2006). Evidence-Based Medicine: A Conceptual Framework. In Haines SJ and Walters BC (Eds.), Evidence-Based Neurosurgery: An Introduction (Pages 1-17). New York: Thieme Medical Publishers.
Results
Facial Nerve Monitoring
Question 1
Does intraoperative facial nerve monitoring during vestibular schwannoma surgery lead to better long-term facial function?
Target population
This recommendation applies to all adult patients undergoing vestibular schwannoma surgery regardless of tumor characteristics.
Recommendation
Level 3: It is recommended that intraoperative facial nerve monitoring be routinely utilized during vestibular schwannoma surgery to improve long-term facial nerve function.
Study Selection and Characteristics
A total of 2853 candidate studies were screened and assessed for eligibility per the previous criteria, and 21 studies were included in the final review.9–29 Postoperative FN function with the use of intraoperative electrically evoked testing with EMG versus facial function in unmonitored surgery was the basis of the recommendations in this section. To be included as a part of this recommendation, a study had to provide a cohort of patients with assessment of pre- and postoperative FN function using an established FN function grading system, such as the HB scale or the SB scale. Furthermore, the method of intraoperative FN monitoring had to be clearly delineated with a comparison between monitored and unmonitored cohorts. Using these criteria, a final total of 3 studies were included for analysis (Table 3A).14,16,25
In cases where an authoring center published multiple papers that met these criteria, only the study with the largest number of subject patients was used to avoid duplicate reporting of patient data if the patient recruitment dates overlapped. Data extraction included study design, level of evidence, number of patients, tumor characteristics, method of ICNM, and long-term FN function.
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
Three studies met the inclusion criteria for this recommendation.14,16,25 All 3 studies represent Class III data, primarily due to lack of blinded assessment and the absence of a validation set. The key results of individual studies are outlined in evidence Table 3A and are summarized within the guideline recommendations. All 3 studies performed a retrospective analysis of postoperative FN function between unmonitored and monitored cohorts.
In 1994, Lenarz and Ernst16 performed a retrospective review of 64 VS patients who underwent microsurgical resection by the same surgeon at a single institution between 1986 and 1991. The goal of the study was to compare postoperative facial function between monitored (n = 30) and unmonitored groups (n = 34). The 2 groups were comparable with respect to tumor size, surgical time, and surgical approach (middle fossa or translabyrinthine). ICNM consisted of facial muscle EMG via needle electrodes, and electrical stimulation of the nerve was performed with bipolar forceps using constant current pulses of 100 microseconds (μs) and current strength between 0.05 and 0.8 milliamps (mA). The average tumor size in the monitored group was 1.5 cm (± 0.5 cm) and in the unmonitored group was 1.7 cm (± 0.7 cm). They could correlate intraoperative tonic (train) activity per hour of surgery, as well as postresection threshold current with immediate postoperative facial function. An increase in train activity and an increase in threshold current (mA) with decreasing wave amplitude at the end of the case correlated with worse immediate facial function. The lack of intraoperative stimulation at the end of the case was predictive of a complete immediate postoperative facial paralysis. The use of monitoring improved immediate and long-term FN outcomes (P < .05). This was especially true in tumors >1.5 cm: HB grade I to II at 1 year 87% (monitored) versus 74% (unmonitored); grade III to VI at 1 year 13% (monitored) versus 26% (unmonitored).
In 1993, Silverstein25 performed a retrospective analysis of 121 VS patients who underwent microsurgical resection by the same surgeon at a single center between 1974 and 1991. Postoperative facial function was assessed immediately and at >1 year in monitored (n = 65) and unmonitored cases (n = 56). Surgery consisted of retrosigmoid and translabyrinthine approaches. EMG facial monitoring was applied using various techniques over the course of the study, in line with advancement in software and hardware developments. Electrical stimulation of the nerve was performed with insulated stimulator probe tips and insulated micro instruments. Electrical pulsed currents ranged from 0.05 to 3 mA. Facial function results were reported for the entire cohort and subanalyzed by surgical approach. Subgroup analysis for surgical approach found no statistical difference between the monitored and unmonitored groups. A distinction was made between the translabyrinthine group, subtotal versus total resection. The authors found statistically worse function after total tumor resection via the translabyrinthine approach when compared to subtotal resection via the translabyrinthine approach or retrosigmoid approach. There were more patients with the FN transected at surgery in the unmonitored group (P < .05). Analysis of the entire cohort found that patients had statistically better facial function in the monitored group than in the unmonitored group (P < .02) immediately and at 1-year follow-up. Assessment of both monitored and unmonitored groups found that large tumors (>3 cm) had poorer FN outcomes when compared to small (<1.5 cm) or medium-sized (1.5–3 cm) tumors (P < .01). This study covers a large span of time (17 years). Improvements in monitoring technology along with increased surgeon expertise over the time span contribute bias to the analysis. In addition, the authors report a recent trend to perform subtotal resection in larger tumors in efforts to preserve the anatomical integrity of the nerve.
In 1991, Kwartler14 performed a retrospective analysis of 244 VS patients who underwent microsurgical resection at a single institution between 1986 and 1987. All patients had tumor resection via the translabyrinthine approach. Eighty-nine patients were monitored, and 155 patients were unmonitored. EMG was measured using bipolar hookwire electrodes in the facial musculature and direct electrical stimulation using a monopolar probe with constant-current stimulus from 0.05 to 3 mA. Monitored patients had a statistically significant better FN outcome in the perioperative period; however, this advantage was not seen at 1 year of follow-up. Subanalysis performed with tumor size found worse facial function in tumors >2.5 cm (P < .01).
Synthesis of Results
Level 3 data suggests the use of ICNM of the FN during VS surgery leads to better facial function outcomes. The 3 studies assessed postoperative facial function in patients undergoing microsurgical resection of VSs with or without use of ICNM. Electrical stimulation offers the ability to help localize and map the course of the FN and may alert the surgeon to stretch injury by way of eliciting train or tonic activity. Larger tumors had an overall worse prognosis for postoperative FN function even with use of FN monitoring. Increased train or tonic activity along with elevated threshold currents following tumor resection were poor prognostic indicators for postoperative FN function.
Discussion
The benefits of ICNM in VS surgery has been widely reported over the last few decades, and again, supported by this analysis. Interestingly, the 3 studies used in this recommendation were published in the 1990s. This reflects the paucity of surgical literature providing direct comparison between monitored and unmonitored surgeries due to the now common use of ICNM during VS tumor resection.
Question 2
Can intraoperative facial nerve monitoring be used to accurately predict favorable long-term facial nerve function after vestibular schwannoma surgery?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery.
Recommendation
Level 3: Intraoperative facial nerve monitoring can be used to accurately predict favorable long-term facial nerve function after vestibular schwannoma surgery. Specifically, the presence of favorable testing reliably portends a good long-term facial nerve outcome. However, the absence of favorable testing in the setting of an anatomically intact facial nerve does not reliably predict poor long-term function and therefore cannot be used to direct decision-making regarding need for early reinneravation procedures.
Study Selection and Characteristics
A total of 2853 candidate studies were screened and assessed for eligibility per the previous criterion and 21 studies were included in the final review.9–29 The electroprognostic value of ICNM in determining good long-term postoperative facial function was the basis of the recommendation in this section. To be included as a part of this recommendation, a study had to provide a cohort of patients with assessment of pre- and postoperative FN function using an established FN function grading system, such as the HB scale or the SB scale. In addition, the method of intraoperative FN monitoring had to be described and a minimum of 1-year follow-up was required for determination of long-term outcomes. There were 15 studies that met the inclusion criteria for this recommendation.9,10,12,17–24,26–29 In cases where an authoring center published multiple papers that met these criteria, only the study with the largest number of subject patients was used to avoid duplicate reporting of patient data if the patient recruitment dates overlapped. Data extraction included study design, level of evidence, number of patients, method of ICNM, electrical characteristics of the ICNM that correlated with postoperative facial function, and assessment of FN function at ≥1 year postoperatively.
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
All 15 studies represent Class III data, primarily due to the lack of blinded assessment and the absence of a validation set. The key results of individual studies are outlined in evidence Table 3B and are summarized within the guideline recommendations. Of the 15 studies used in this analysis, 12 studies9,10,17–21,23,24,26–28 identified intraoperative eletrophysiologic parameters that were predictive of “good” postoperative facial function at ≥1 year. Good function in this analysis was defined as HB I-II. Heterogeneous electroprognostic parameters were used between studies; however, all authors provided details on the criteria applied for their assessments. All the ICNM techniques made use of continuous or electrically evoked EMG activity.
In 2013, Schmitt et al23 described a decade of experience with the use of monopolar pulsed constant-current stimulation at supramaximal levels that were tested medial and distal to tumor resection. These 2 measurements were used to create an amplitude ratio, which is reported as a percent dropoff. A percent dropoff of ≤69% yielded a predictive value of 94% for postoperative HB I-II function. This method was not reliable in predicting poor functional outcomes and marginal in predicting moderate function.
Also in 2013, Arnoldner et al10 reported on the predictive value of using percent maximum values, defined as current level stimulus/maximum muscle response (see Table 3 for specifics). A percent maximum of >50 had a 0.9 PPV for HB I-II function. The responses obtained with 0.3-mA current at the brainstem yielded the best predictive results for HB I-II function compared to the other studied currents of 0.05, 0.1, and 0.2 mA. The authors recommended this monitoring method as complementary when evoked responses do not conform to more conventional predictors.
Marin et al’s 2011 study19 described 100% success in determining HB I-II outcome at 1 year after surgery when the brainstem stimulation threshold was <0.05 mA. This dropped to 93% if the threshold was 0.05 mA. Also in 2011, Amano et al9 reported a high predictive value by using amplitude ratios gathered from continuous evoked EMG (refer to Table 3 for formula specifics). This method was heralded as a real-time assessment able to facilitate warning criteria that could influence the surgeon to stop tumor dissection. An amplitude preservation ratio of >50% had a 95% probability of maintaining HB I-II at 1 year. The biggest limitation with this method would be identifying the FN root at the start of surgery to place the probe, something that may not be possible with larger tumors. Once in place, there is a need to repeatedly check for probe migration (the authors verified probe position a minimum of every 30 minutes). This type of monitoring requires a demanding continuous assessment and interpretation of the various compound muscle action potentials (CMAPs) by an experienced electrophysiologist. Finally, facial muscle groups were assessed individually as opposed to the more conventional composite assessment of facial function (results reported for 558 muscles in 216 patients). The concept of using A-train time, a reflection of neurotonic discharge activity, as a prognosticator of facial function has also been reported. In 2007, Prell et al22 found that an A-train time longer than 10 seconds was correlated with long-term deficits in facial function with a specificity of 81%. For the patients with A-train times of <10 seconds and normal preoperative facial function, 81% regained normal function at 1 year. Amplitude at the minimum stimulus threshold (MST) was used by Neff et al21 as a prognostic indicator of function at 1 year after surgery. Applying a logistic regression model, the probability of achieving HB I-II was 98% when MST was ≤0.05 mA and response amplitude was >240 µV. Independently, the 2 parameters were not as sensitive. The authors cautioned that amplitude results were technique-dependent, with responses varying according to the contact established between probe and the FN. In 2002, a study by Nakao et al20 found that ordinary or irritable patterns on EMG during the last step of tumor removal predicted 85% and 95% HB I-II function at 1 year, respectively. The last step was in reference to dissection of tumor around the porus of the internal auditory canal, which they leave for last. Silent patterns, on the other hand, were more likely associated with poor long-term outcome (HB III-VI).
In 2002, Fenton et al12 provided a follow-up on a prior report on the utility of using the minimum stimulation intensity medial to the tumor after excision (MIMAE) and facial function at 2-year follow-up. Consistent with their prior report,13 MIMAE was again not found to be an independent predictor of long-term facial function. Another studied parameter that ultimately lacked electroprognostic value involved amplitudes responses in evoked facial muscle responses when the FN was stimulated at the brainstem. Yokoyama et al29 described how this method was better at predicting time to recovery rather than ultimate functional outcome. Mandpe et al18 found that by combining postresection stimulation thresholds and response amplitudes (these were obtained distal and proximal to tumor resection), the 2 had superior prognostic value than when they were used independently. By using these 2 parameters, they had a 12% false positive rate when predicting good HB function. Magliulo et al17 compared 3 previously reported electroprognostic methods (see Table 3 for specifics) and found the most reliable to be ratios of stimulation intensity over the amplitude evoked responses when compared to amplitudes of train activity or amplitude of evoked response at the brainstem postresection of tumor. The ratios were helpful in predicting HB I-II outcomes at 1 year; however, they were not reliable in predicting poor outcomes. The study was limited by small size and retrospective analysis. A prospective analysis of 109 patients by Zeitouni et al28 in 1997 found good prognostic value in the minimum stimulus thresholds obtained at the brainstem post-tumor resection. A stimulus threshold of <0.1 mA predicted good facial function at 1 year in 87% of their cohort. Conversely, higher thresholds were not predictive of poor outcomes. Selesnick et al24 grouped meningioma and VS patients in a retrospective study in 1996. A stimulation threshold of ≤0.2 mA was predictive of good long-term facial function. Again, poor function was not reliably measured using this parameter. The results do not differentiate between the 2 different pathologies included in the study cohort, VS and meningioma. The authors mention that meningiomas comprised 14% of tumors, but no further differentiation in electrical activity was reported between the 2 groups. Taha et al27 used amplitude ratios measured postresection at the brainstem and distally at the internal auditory canal, and determined that a ratio of 2:3 was predictive of good long-term function. Statistical analysis, however, was not reported, and the study cohort was small at 20 patients. Silverstein et al26 used the minimum current levels needed to elicit a response at the brainstem after tumor resection and found a strong correlation with good facial function when responses were elicited at ≤0.1 mA. The poor outcomes, however, could not be predicted. Lalwani et al15 concluded that good long-term FN function correlated well with thresholds of 0.2 volts (V) or less at the brainstem, posttumor resection.
Synthesis of Results
Level 3 data suggest wide variability in ICNM protocols with multiple different electroprognostic parameters found to accurately predict good long-term postoperative FN function following VS surgery. Successful parameters included postresection stimulation currents or thresholds, response amplitudes, and continuous EMG patterns. Ratios using a combination of these parameters were also successfully applied. Of the various stimulating probes used for direct nerve stimulation, monopolar devices were preferred or reported as most consistent by various groups.9,11,12,15,17–19,23,24,26–30
Discussion
Evidence suggests that various methods can be successfully used to predict good postoperative FN function following VS resection. While several electroprognostic parameters were identified as positive predictors of good functional outcome, none of them could consistently predict poor long-term function. The lack of consistency in methods by authors was driven by institutional experience, comfort level of the surgical team, availability of specific equipment, and ultimately, the presence of an independent electrophysiology service. Continuous EMG monitoring, such as when evaluating for tonic or train activity, is laborious and requires a dedicated team member for continuous assessment throughout tumor resection. This is also a task that requires a specific skillset for interpretation. Other methods, such as postresection thresholds at the brainstem, are not as laborious; however, even these measurements are afflicted by confounding factors, such as variability in equipment and their specific electrical settings. The desired benefit of using electroprognostic parameters to predict good functional outcome lies in the ability to counsel patients on the timing of surgical intervention for facial rehabilitation. An observation period of 12 to 18 months is typically adhered to in patients with postoperative paresis or paralysis and anatomically intact FN to allow for spontaneous return of function. If a reliable electrical parameter predictive of ultimate good facial outcome is possessed, the clinician can confidently counsel patients to proceed with conservative management and postpone early surgical dynamic facial rehabilitation. Conversely, none of the parameters proved to successfully predict poor functional outcome. This is a reflection on the limitation of electrical currents in distinguishing neuropraxia from axonotmesis or neurotmesis at a single time point, following resection.
Question 3
Does an anatomically intact facial nerve with poor electromyogram electrical responses during intraoperative testing reliably predict poor long-term facial nerve function?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery.
Recommendation
Level 3: Poor intraoperative electromyogram electrical response of the facial nerve should not be used as a reliable predictor of poor long-term facial nerve function.
Study Selection and Characteristics
A total of 2853 candidate studies were screened and assessed for eligibility per the previous criterion and 21 studies were included in the final review.9–29 The electroprognostic value of ICNM in determining poor long-term postoperative FN function was the basis of the recommendation in this section. To be included as a part of this recommendation, a study had to provide a cohort of patients with assessment of pre- and postoperative FN function using an established FN function grading system, such as the HB scale or the SB scale. In addition, the method of intraoperative FN monitoring had to be described and a minimum of 1 year of follow-up was required for the determination of long-term outcomes.
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
All studies were thought to represent Level 3 data, primarily due to the lack of blinded assessment and the absence of a validation set. The key results of individual studies are outlined in evidence Table 3C and are summarized within the guideline recommendations. Of the 21 studies used in this analysis, 4 studies discussed intraoperative eletrophysiologic parameters with electroprognostic value for “poor” postoperative facial function at ≥1 year.9,11,20,22 Poor function in this analysis was defined as HB grade IV or greater. There was a heterogeneous methodology used in electroprognostic parameters; however, all authors provided details on the criteria applied for their assessment. All the ICNM techniques made use of continuous or electrically evoked EMG activity. Most studies listed below were described in detail in the earlier sections of this paper; therefore, only highlights pertaining to the question at hand will be included in this section.
In 2012, Carlson et al11 specifically evaluated long-term facial outcomes in patients with poor electrical response after tumor resection in anatomically intact nerves. They could effectively prove that absence of electrical response did not definitively imply poor functional outcome, which was defined as HB IV-VI. Although the study cohort was small, at 11 patients, only 36% of the patients with electrical silence ultimately developed poor function and 18% of patients (n = 2) reached HB II status. These results speak against committing patients to immediate intraoperative FN grafting because of the possibility for spontaneous recovery. In 2011, Amano et al9 used a logistic regression analysis of amplitude preservation ratios as a risk assessment tool for surgeons. Ratios of <40% carried a higher risk of poor facial function at 1 year and was the authors’ own personal indicator to stop tumor resection to reduce the chances of a severe facial palsy. This indicator, however, did not seem to be part of a strict protocol, and further details or statistical analysis were not provided. Duration of A-train activity was a negative predictive factor as discussed by Prell et al22 in 2007. A-train time >10 seconds was associated with a minimum of a 2-grade drop in HB function in the early and late postoperative period (P < .001 and P < .015, respectively). A sensitivity of 57.1% and specificity of 81% was reported for poor long-term facial outcomes. In 2002, Nakao et al20 found that silent patterns on EMG were predictors of poor facial function; however, this was also based on a very small cohort (2/11 patients or 11%).
The remaining studies were unable to identify reliable independent parameters for poor long-term functional outcomes. This included supramaximal stimulation (SMS) of proximal to distal ratios,23 the concept of percent of maximum (current stimulus/maximum muscle response),10 maximum stimulus thresholds (MST),21,26 minimum stimulation thresholds (ST),19,24,28 minimum stimulation intensity after tumor excision (MIMAE),12 or voltage of evoked amplitudes15,29 and a combination or ratios of the response thresholds and amplitudes.17,18,27
Synthesis of Results
Level 3 evidence suggests that A-train duration, amplitude ratios, absent electrical responses, and silent EMG patterns are potential prognosticators for poor facial function outcomes. Although a silent pattern or A-train EMG activity were prognostic indicators for poor function, patients with A-train activity and even absent electrical stimulation after tumor resection were also shown to still have opportunity for spontaneous recovery in the long term. Therefore, the absence of electrical stimulation after tumor resection does not necessarily commit patients to permanent facial paralysis.
Discussion
Level 3 data do not support the use of specific electroprognostic criteria to reliably predict poor facial function after VS surgery. Although a handful of parameters were presented as potential predictors, none had strong predictive value or were powered to do so. The strongest argument against using electrical markers as predictors for poor function was based on observation that patients with electrical silence, or absent responses at the end of surgery, did not necessarily develop a permanent facial paralysis. Whereas several markers can be reliably used to predict good facial function, the ability to predict poor function is still limited. Because we cannot reliably predict poor long-term FN function with intraoperative electroprognostic testing, early facial reanimation should not be employed unless nerve transection is certain.
Cochlear Nerve Monitoring
Question 4
Should intraoperative eighth cranial nerve monitoring be used during vestibular schwannoma surgery?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery with measureable preoperative hearing levels and tumors <1.5 cm.
Recommendation
Level 3: Intraoperative eighth cranial nerve monitoring should be used during vestibular schwannoma surgery when hearing preservation surgery is attempted.
Study Selection and Characteristics
A total of 1849 candidate studies were screened and assessed for eligibility per the previous criterion and 7 studies were included in the final analysis.31–37 The value of ICNM in hearing preservation was the basis of the recommendation in this section. In order to be included as a part of this recommendation, a study had to provide a cohort of patients with assessment of pre- and postoperative hearing function using an established system, such as the 1995 AAO-HNS or the GR hearing classification system, or presented data using WRS and PTA for defining hearing status, or had individual patient data presented such that the latter criteria could be applied and analyzed. In addition, the method of intraoperative cochlear nerve monitoring had to be described. Data extraction included study design, level of evidence, number of patients, tumor characteristics, method of ICNM, and the electrical characteristics that correlated with postoperative hearing function.
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
All studies were thought to represent Level 3 data, primarily due to the lack of blinded assessment and the absence of a validation set. The key results of individual studies are outlined in evidence Table 4A and are summarized within the guideline recommendations. Of the 7 studies noted above, 5 studies provided objective comparisons between monitored and unmonitored surgeries31,33–36 and are therefore used in this recommendation. Hearing preservation in this analysis was defined as any measurable hearing using the AAO-HNS or GR classification systems. Each of the 5 studies will be described briefly.
In 2008, Piccirillo et al31 retrospectively reviewed hearing outcomes in patients with tumors <1.5 cm and normal preoperative hearing. They did not find an advantage in hearing preservation outcomes when comparing monitored versus unmonitored cases. A significant prognostic factor, however, was the presence of cranial nerve action potentials (CNAP) at the end of surgery. Those patients were statistically more likely to have good (AAO-HNS Class A) postoperative hearing (P < .01). The presence of CNAP at the end of surgery, however, did not ensure good hearing outcomes. In their series, more than half of the patients with intact CNAP after tumor removal ultimately had poor hearing outcomes. The technical difficulties of direct eighth nerve monitoring (DENM), such as (1) initial placement of the electrode proximal to the tumor and (2) maintaining that placement throughout surgery, were highlighted and are important considerations for surgeons wishing to undertake this type of monitoring. Finally, a limitation is the lack of long-term data. The authors do not delineate the timeline in which postoperative hearing function was assessed, thereby, limiting assessment of long-term outcomes.
In 1994, Nedzelski et al33 assessed cochlear compound action potentials (CAP) as an electroprognostic parameter for hearing preservation. Of the 80 patients included in the cohort, 56 were successfully monitored. This was a retrospective review that compared monitored (n = 56) to unmonitored cases (n = 20). All patients had preoperative serviceable hearing and tumors ≤1.5 cm. Long-term hearing assessments were provided at 1 year after treatment and hearing preservation rates were higher in the monitored group (P < .02). Significantly better hearing preservation rates were seen in patients with a measurable intraoperative CAP following tumor resection, although 1 patient with absent CAP had serviceable hearing. CAP click threshold shifts of ≤20 dB predicted serviceable hearing levels in 71% of patients. Shifts >20 dB, in turn, predicted poor hearing outcomes. Eighteen of the patients with measureable CAP either had absent or nonserviceable hearing, which speaks to the inconsistency of this parameter. This is thought to be secondary to the persistence of cochlear microphonic potentials in the distal cochlear nerve despite anatomic discontinuity or dysfunction in the proximal segment.
In 1992, Harper et al35 experienced significant improvement in hearing preservation rates in monitored cases using ABR. The difference was statistically significant only for small tumors (≤1.1 cm). In their experience, preservation of Wave I and V were positive prognostic factors, with a 67% likelihood of useful hearing preservation. Postoperative hearing was measured at 3 months, limiting long-term assessment. Similar findings were reported by Slavit et al in 1991.36 In this study, a comparison was made between ABR-monitored cases versus no monitoring. Although there was no statistical advantage in the ABR group, there was a trend in that direction. The most pronounced effect was seen in tumors that were <1 cm, and none of the patients with tumors >3 cm had preservation of hearing. Kemink et al37 found that complete loss of ABR Wave V was predictive of profound hearing loss. However, not all patients with complete hearing loss had an absence of Wave 5. In this cohort, hearing preservation was not achieved in patients with tumors >1.5 cm. In a smaller cohort, Kveton et al34 did not find a significant difference between monitored and unmonitored cases. On the contrary, the study showed improved serviceable hearing preservation (AAO-HNS Class C or better) in the unmonitored group compared to those monitored with ABR. However, this was not a significant difference. In addition, correlation with tumor size or preoperative hearing levels was not provided.
Synthesis of Results
Level 3 evidence supports the use of intraoperative cochlear nerve monitoring in hearing preservation VS surgery. The most common method employed was ABR. The presence or characteristics of Wave I and V, as well as the CAP, were the most useful parameters discussed. The benefit of monitoring was most pronounced in tumors <1.5 cm. Hearing preservation in tumors >3 cm was not observed. Long-term assessments were not uniform, with several groups reporting hearing levels measured only 3 months after treatments or not reporting timing at all. Such short-term assessments limit the ability to assess permanent function.
Question 5
Is direct eighth cranial nerve monitoring superior to the use of far-field auditory brain stem responses?
Target population
This recommendation applies to adult patients undergoing vestibular schwannoma surgery with measurable preoperative hearing levels and tumors <1.5 cm.
Recommendation
Level 3: There is insufficient evidence to make a definitive recommendation.
Study Selection and Characteristics
A total of 1849 candidate studies were screened and assessed for eligibility per the previous criteria, and 7 studies were included in the final analysis.31–37 The utility of ICNM in hearing preservation was the basis of the recommendation in this section. A focus on 2 specific modalities, DENM and far-field ABR, was addressed. To be included in this recommendation, a study had to provide a cohort of patients with assessment of pre- and postoperative hearing function using an established system, such as the AAO-HNS or GR hearing classification system, or presented data using WRS and PTA for defining hearing status, or had individual patient data presented such that the latter criteria could be applied and analyzed. In addition, the method of intraoperative cochlear nerve monitoring had to be described and direct comparison between DENM and ABR provided. Of the seven studies noted above, one study met the inclusion criteria for this recommendation.32 Data extraction included study design, level of evidence, number of patients, tumor characteristics, method of ICNM, electrical characteristics evaluated, and pre- and postoperative hearing levels.
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
The study used for this recommendation was thought to represent Level 3 data, primarily due to the lack of blinded assessment and the absence of a validation set. The key results of the study are outlined in Table 4B and summarized within the guideline recommendations. This was the only study that provided a direct comparison between the 2 modalities of cochlear nerve monitoring.
In 2004, Danner et al32 retrospectively compared hearing preservation outcomes between the use of DENM and ABR. In their series, DENM offered improved hearing preservation outcomes when compared to ABR. The authors attributed superiority to the larger amplitudes obtained with DENM, which in turn required less data averaging and translated into faster, “real-time” assessment of nerve integrity. There was a bias in choice of monitoring modality in that DENM became the preferred monitoring modality after 1995 (study range 1992–2002). They felt experience bias did not affect outcomes in this comparison because of the senior surgeon’s established expertise at the onset of the study. It was the senior surgeon’s opinion that his learning curve had plateaued at the onset of the study, which is a subjective assessment with risk for recall bias. Consistency in their surgical technique between ABR and DENM was emphasized and discussed to mitigate suspected experience bias. The numbers, however, are skewed toward the DENM group, which was double the size of ABR group, 44 versus 22 patients, respectively. Hearing preservation rates were overall highest amongst patients with tumors ≤1.5 cm, regardless of monitoring modality. Again, long-term hearing outcomes were not assessed, and the timing of postoperative hearing assessments was not specified.
Discussion for Cochlear Nerve Section
The challenges of defining “hearing preservation” continue to plague the literature. Hearing preservation rates vary with respect to the criteria used to report them. The variability has been addressed by endorsing standardized hearing classification systems, such as the AAO-HNS or GR scales. Despite these efforts, consensus lacks on what characterizes useful or serviceable hearing. In the AAO-HNS system, Class A and Class B represent “useful” or “serviceable” hearing and constitute successful hearing preservation surgery. The equivalent in the GR scale is represented by grades I and II. Due to the variability in the reports surrounding what can be classified as successful hearing preservation, the authors opted to be inclusive of hearing levels, not just serviceable hearing, as long they were reported using a standardized system or provided PTA or WRS levels. Applying the aforementioned serviceable hearing criteria to the entire analysis would have been too restrictive given the limited number of studies available for this article. The questions posed in the cochlear nerve section will be discussed in tandem as they include only 7 studies, compared to the larger amount of literature available for the FN section.
The data extracted from modern day reports supported using ICNM for hearing preservation in patients with preoperative hearing and small tumors. The benefit was seen with ABR or DENM. A tumor size cutoff of ≤1.5 cm was identified as being more likely to provide hearing preservation than larger tumors. Hearing outcomes in larger tumors were poor regardless of preoperative hearing status or monitoring modality. The biggest challenge with neuromonitoring of the cochlear nerve involves the technical aspects and delayed feedback. ABR is plagued by delay issues due to the data averaging that is required to assess changes in function. To circumvent this, direct cochlear nerve monitoring has been used instead. The technical requirements and challenges of performing direct cochlear nerve monitoring, however, were made apparent in various reports. They range from the inability to place electrodes at the nerve root exit zone prior to tumor resection to the difficulty in keeping the probes in place throughout the duration of surgery or securing the probe without causing iatrogenic damage to the nerve. Finally, factors such as the presence of excess cerebrospinal fluid or blood, the stimulation voltage used to elicit responses, or the interference of electrocautery stimuli have all been reported to alter responses and the interpretation of results. Dedicated, well-trained electrophysiologists are important members of a hearing preservation team, and most will argue are a necessity.
In summary, ICNM monitoring has a role in hearing preservation VS surgery. Although there are limitations, Level 3 evidence supports its use. When available, direct eighth nerve monitoring should be employed as well, or in addition to ABR, because of the more immediate real-time responses that can potentially alert the surgeon to noxious stimuli or manipulations. Not all centers have the capability to perform DENM or the electrophysiologists to properly interpret the information during surgery, which limit its widespread implementation. It should also be highlighted that our assessment of the superiority of DENM in hearing preservation surgery is based on 1 study, and, as such, caution is advised in implementing drastic neuromonitoring changes to an already successful surgical team. More studies and data are needed to better assess this electrical modality.
Conclusion and Key Issues For Future Investigations
The goals of VS surgery have shifted over the years. The safety profile of these surgeries has continued to improve, and modern-day mortality is at an all-time low. As a result, a great deal of focus is now placed on minimizing morbidity, including hearing loss and facial paresis. The current expectation is that complete tumor resection is to be undertaken with a serious intent to achieve good postoperative facial function. A similar concept has been adopted in patients with existing preoperative hearing. Although the primary goal of VS surgery is still to achieve safe and complete tumor resection, a shift into subtotal resections with the hope of preserving these 2 functions has become more widely accepted. The benefits of using ICNM has been accepted and is supported in this analysis. Despite the best of surgical techniques and electrophysiology equipment, surgical outcomes are still bound by tumor characteristics, such as size. Large tumors are more likely to result in facial paralysis and hearing loss when compared to small tumors.
As technology continues to evolve and the comfort level of surgical teams continues to improve, clinicians will hopefully learn more about specific parameters that will help as reliable prognosticators of functions. Although several factors were discussed in this review, the sensitivity and specificity profile of each will need to be validated and reproduced in future studies. More prospective analyses will be needed to help with this endeavor.
Conflict of Interest (COI)
The Vestibular Schwannoma Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript (here).
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient’s physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Committee for its review, comments, and suggestions throughout peer review, as well as Trish Rehring, MPH, CHES, and Mary Bodach, MLIS, for their assistance. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Sepideh Amin-Hanjani, MD, D. Ryan Ormond, MD, Andrew P. Carlson, MD, Kimon Bekelis, MD, Stacey Quintero Wolfe, MD, Chad W. Washington, MD, Cheerag Dipakkumar Upadhyaya, MD, and Mateo Ziu, MD
Figures
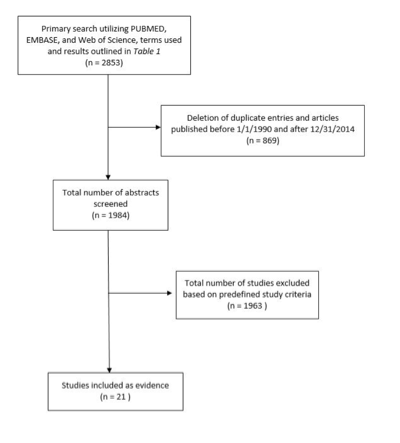
Figure 1. Facial nerve monitoring article flow chart.
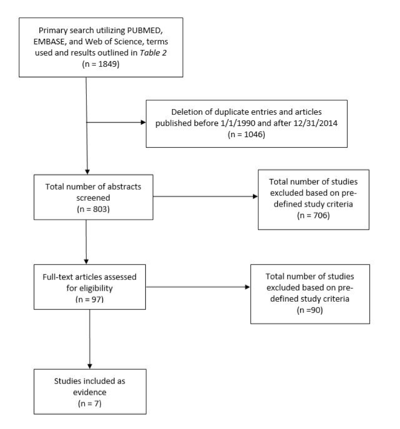
Figure 2. Cochlear nerve monitoring article flow chart.
Table 1. Facial nerve monitoring primary search strategy, results, and initial pruning
|
ENDNOTE PUBMED (NLM), searched on May 10, 2015:
|
|
Search 1: All Fields, Contains “Acoustic neuroma” AND, all fields, contains, “Facial nerve” AND, all fields, contains “Surgery”
Total 1392
|
|
Search 2: All Fields, Contains “Vestibular schwannoma” AND, all fields, contains “Facial nerve” AND, all fields, contains “Surgery”
Total 676
|
|
Search 3: All Fields, Contains “Acoustic neuroma” AND, all fields, contains, “Facial nerve” AND, all fields, contains “Prognostic”
Total 58
|
|
Search 4: All Fields, Contains “Vestibular schwannoma” AND, all fields, contains “Facial nerve” AND, all fields, contains “Prognostic”
Total 35
|
|
TOTAL: 2161
TOTAL with duplicates excluded: 1519
|
|
ENDNOTE EMBASE, searched on May 10, 2015:
|
|
Search 1: Abstract, Contains “Acoustic neuroma” AND, abstract, contains, “Facial nerve” AND, abstract, contains “Surgery
Total 207
|
|
Search 2: Abstract, Contains “Vestibular schwannoma” AND, abstract, contains “Facial nerve” AND, abstract, contains “Surgery”
Total 233
|
|
Search 3: Abstract, Contains “Acoustic neuroma” AND, abstract, contains, “Facial nerve” AND, abstract, contains “Prognostic”
Total 12
|
|
Search 4: Abstract, Contains “Vestibular schwannoma” AND, abstract, contains “Facial nerve” AND, abstract, contains “Prognostic”
Total 24
|
|
TOTAL 476
TOTAL with duplicates excluded: 432
|
|
ENDNOTE Web of Science, searched on May 10, 2015:
|
|
Search 1: Title/Keywords/Abstract, contains “Acoustic neuroma” AND, Title/Keywords/Abstract, contains, “Facial nerve” AND, Title/Keywords/Abstract, contains “Surgery”
Total 732
|
|
Search 2: Title/Keywords/Abstract, contains “Vestibular schwannoma” AND, Title/Keywords/Abstract, contains “Facial nerve” AND, Title/Keywords/Abstract, contains “Surgery”
Total 599
|
|
Search 3: Title/Keywords/Abstract, contains “Acoustic neuroma” AND, Title/Keywords/Abstract, contains, “Facial nerve” AND, Title/Keywords/Abstract, contains “Prognostic”
Total 78
|
|
Search 4: Title/Keywords/Abstract, contains “Vestibular schwannoma” AND, Title/Keywords/Abstract, contains “Facial nerve” AND, Title/Keywords/Abstract, contains “Prognostic”
Total 65
|
|
TOTAL 1474
TOTAL with duplicates excluded: 902
|
|
Summary of primary search: facial nerve monitoring
Combined from 3 database searches, total of 2853 candidate articles
Deleted articles published before 1/1/1990 and after 12/31/2014.
Deleted all duplicate articles
Total number of candidate articles after primary search = 1984
|
Table 2. Cochlear nerve monitoring primary search strategy, results and initial pruning
|
ENDNOTE PUBMED (NLM), searched on May 10, 2015:
|
|
Search 1: All Fields, Contains “acoustic neuroma” OR All fields, Contains “vestibular schwannoma” AND All Fields, Contains “audiometric”
Total: 176
|
|
Search 2: All Fields, Contains “acoustic neuroma” OR All fields, Contains “vestibular schwannoma” AND All Fields, Contains “tinnitus”
Total: 456
|
|
Search 3: All Fields, Contains “acoustic neuroma” OR All fields, Contains “vestibular schwannoma” AND All Fields, Contains “sudden hearing loss”
Total: 183
|
|
Search 4: All Fields, Contains “acoustic neuroma” OR All fields, Contains “vestibular schwannoma” AND All Fields, Contains “asymmetry”
Total: 68
|
|
TOTAL: 883
|
|
ENDNOTE EMBASE, searched on May 10, 2015:
|
|
Search 1: Abstract, Contains “acoustic neuroma” OR Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “audiometric”
Total: 108
|
|
Search 2: Abstract, Contains “acoustic neuroma” OR Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “tinnitus”
Total: 253
|
|
Search 3: Abstract, Contains “acoustic neuroma” OR Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “sudden hearing loss”
Total: 37
|
|
Search 4: Abstract, Contains “acoustic neuroma” OR Abstract, Contains “vestibular schwannoma” AND Abstract, Contains “asymmetry”
Total: 40
|
|
TOTAL: 438
|
|
ENDNOTE Web of Science, searched on May 10, 2015:
|
|
Search 1: Title/Keywords/Abstract, Contains “acoustic neuroma” OR Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “audiometric”
Results: 112
|
|
Search 2: Title/Keywords/Abstract, Contains “acoustic neuroma” OR Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “tinnitus”
Results: 243
|
|
Search 3: Title/Keywords/Abstract, Contains “acoustic neuroma” OR Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “sudden hearing loss”
Results: 124
|
|
Search 4: Title/Keywords/Abstract, Contains “acoustic neuroma” OR Title/Keywords/Abstract, Contains “vestibular schwannoma” AND Title/Keywords/Abstract, Contains “asymmetry”
Results: 49
|
|
TOTAL: 528
|
|
Summary of primary search: cochlear nerve monitoring
Combined from 3 database searches, total of 1849 candidate articles
Deleted all duplicate articles
Total number of candidate articles after primary search = 803
|
Table 3A. Evidence table for question 1
|
Author/Year
|
Study Description
|
Data Class
|
Conclusion
|
|
Lenarz et al, 1994
|
Retrospectively compared FN preservation rates of monitored (n = 30) vs. unmonitored (n = 34) VS patients. Compared immediate and 1-year facial outcomes (HB) between the 2 groups. Both bipolar and monopolar probes used.
Single center, same surgeon experience between 1986 and 1991. NF status not reported.
HB grading system used.
|
III
|
The use of monitoring improved immediate and long-term facial nerve outcomes (P < .05). This was especially true in large tumors >1.5 cm. HB grade I-II at 1 year 87% (monitored) vs. 74% (no monitor). Grade III-VI at 1 year: 13% (monitored) vs. 26% (no monitor).
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients. Experience bias: same surgeon; monitoring cases occurred in later years compared to unmonitored cases.
|
|
Silverstein et al, 1993
|
Retrospective analysis of 121 VS patients. Compared FN outcome immediate and at >1 year (modified HB score) in monitored (n = 65) vs. unmonitored cases (n = 56).
Single center, single surgeon experience between 1974–1991. NF status not reported.
HB grading system used.
|
III
|
There were a statistically greater number of patients with intraoperative eighth nerve transection in the unmonitored group (P < .05). Monitored patients had better overall early and late facial function compared to unmonitored patients (P < .02).
Classification of evidence on prognosis, class III. Did not blind outcome measure. No validation in an independent “test set” of patients. Experience bias since monitoring became available later in surgeon experience.
|
|
Kwartler et al, 1991
|
Retrospective analysis of monitored translabyrinthine VS cases (n = 89) to an unmonitored translabyrinthine VS group (n = 155). Looked at short-term and >1-year facial function outcomes (HB score). Monopolar probe used.
Single center experience between 1986–1987. NF status not reported.
HB grading system used.
|
III
|
Facial nerve outcomes were better at all time points in the monitored group (P < .05) (immediate, time of discharge, 1 year). They found it particularly useful in the tumors >2.5 cm.
Classification of evidence on prognosis, class III. Did not blind outcome measure. No validation in an independent “test set” of patients. Experience bias because monitoring became available later in the surgeon’s experience.
|
FN, facial nerve; HB, House–Brackmann; NF, neurofibromatosis; VS, vestibular schwannoma.
Table 3B. Evidence table for question 2
|
Author/Year
|
Study Description
|
Data Class
|
Conclusion
|
|
Schmitt et al, 2013
|
Retrospective review of facial nerve function outcome after VS resection using SMS proximal to distal dropoff ratio to predict facial nerve function at >1 year postoperatively. Monopolar Prass probe used.
The dropoff ratio was calculated: 1 − {distant response (µV)/proximal response(µV)} × 100%
172 VS patients analyzed with SMS data and >1 year follow-up. Only patients with anatomically intact nerves were included.
NF2 patients were included. Single center and single surgeon experience from 1999–2011.
HB grading system used.
|
III
|
SMS proximal to distal dropoff ≤69% at the end of surgery has 94% chance of predicting HB I-II. SMS >69% had a 56% chance of HB I-II.
Half the patients with >75% dropoff will still go on to have HB I-III, therefore poor predictor of long-term poor function.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Arnoldner et al, 2013
|
Prospective study. Calculated % maximum (level current stimulus/maximum muscle response) stimulation to predict facial nerve function at >1 year.
%Max = SL/Mmax
After skin closure, the facial nerve was stimulated transcutaneously at the stylomastoid foramen. Increasing stimulus intensities were used until the muscle response amplitude reached a plateau; a supramaximal stimulus was then further applied. The resulting muscle response amplitude was considered the MMax.
Kartush bipolar stimulator was used.
78 VS patients with minimum 1 year follow-up and average follow-up of 523 days. Single center experience between 2005–2010. NF2 patients excluded.
HB grading system used.
|
III
|
%Max calculated using a direct stimulus of 0.3 mA at the brainstem yielded the best predictive results of HB I-II. The facial nerve was stimulated at root exit zone with increasing stimulus intensities 0.05, 0.1, 0.2, and 0.3 mA.
%Max >50 had PPV of HB I-II of 0.9. Sensitivity and specificity was 0.61/0.8, respectively.
For %Max of >40 PPV 0.87; >30 PPV 0.80; >20 PPV 0.80; >10 0.79. Cannot predict poor outcomes; if you get a “poor” response of %Max of 11%; you still have a high chance (79%) of good outcome.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Marin et al, 2011
|
Retrospective analysis of 206 VS patients. Used stimulation threshold of 0.05 mA to predict long-term (1-year) facial nerve function. Monopolar probe used dose stimulation.
Single center and multiple surgeon experience from 1996–2008. NF2 patients excluded. Patients with abnormal facial function preoperatively were excluded.
HB grading system used.
|
III
|
The facial nerve was electrically stimulated at the brainstem by a monopolar probe with a 0.5 mm tip after tumor removal. Stimulation threshold of <0.05 mA predicted HB I-II function in 100% of patients (P < .01). A stimulation threshold of 0.05 predicted HB I-II in 93%. A threshold of >0.05 predicted HB I-II in 82%. Cannot reliably predict poor outcomes with this method.
A response was defined as >100 µV.
Classification of evidence on prognosis, level III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Amano et al, 2011
|
Retrospective review of continual stimulation evoked facial nerve EMG. Calculated an amplitude preservation ratio (%) during and after tumor resection and evaluated whether this could predict long-term facial function at >1 year.
The facial nerve was electrically stimulated with monopolar current 0.1–3 mA at 1 Hz and CMAP continuously monitored. Free running spontaneous EMG, as well as evoked facial EMG were used. The stimulation was via monopolar probe placed at the nerve’s root exit zone at the brainstem.
The amplitude preservation ratio (%) = {last M-max(µV)/Control M-max (µV)} × 100
Control M-max = maximum CMAP amplitude at start of surgery
Total patient sample of 232 consecutive VS patients excluding 16 patients with preoperative facial weakness, prior surgery, or radiation (n = 216).
Single center experience from 2005-2008. NF2 patients excluded.
HB grading system used.
|
III
|
Concluded that continual stimulation evoked facial nerve EMG could be used to determine if tumor resection should continue. An amplitude preservation ratio >50% or last amplitude measured (Mmax) > 1000 µV was predictive of good facial function. A ratio >50% had 95% HB I or II. Unclear what >1000 µV predicted. A discrepancy with µV criteria is that they reached this cutoff with several tumors with a large amount of remnant; thus continued to operate.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Prell et al, 2007
|
Retrospective review of whether A-train duration measured from free running EMG could predict facial nerve function at 1 year postoperatively.
A-train activity is defined as a close succession of ≥4 geometrically
similar, mono- to triphasic discharges from baseline
with an amplitude of at least double background noise. The sequence of ≥4 elements is required to build a frequency of 100–200 Hz, which must be steady over the
course of any given A train. Train time is reported in seconds.
40 VS patients with a minimum of 1-year follow-up. 26 had normal preoperative facial function and 14 did not.
Single center experience from 1994–2003. NF2 status not reported.
HB grading system used.
|
III
|
For patients with normal preoperative function and A-train time <10 seconds, 81% had normal function at 1 year. For the entire cohort, an A-train time of >10 seconds predicted a HB II–VI (everyone but normal HB I) in 81% at 1 year of follow up. 5 of 40 (13%) with prolonged A-train times still became HB I. Sensitivity
was 57.1% and specificity 81% for the 10-second threshold.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Neff et al, 2005
|
Prospective evaluation of 74 consecutive VS patients. Used MST and response amplitude (at MST) to predict long-term facial function at >1 year.
Results report data on 71 patients with postoperative anatomically intact facial nerves. Measurements were made at the brainstem or medial to tumor resection.
Single center experience, date ranges and NF2 status not reported.
HB grading system used.
|
III
|
Using their logistic regression model, an MST ≤0.05 mA with a response amplitude >240 µV carried a 98% probability of HB I–II at 1 year. Patients with MST >0.05 threshold OR <240, or both still obtained HB I–II in 59% (10/17). P =.015.
Predicting poor outcome was not as reliable, perhaps because of the small number of patients in this category (HB III–VI).
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Nakao et al, 2002
|
Prospective analysis of EMG “pattern” (irritable, silent, stray, ordinary) during the critical portion of tumor removal to see if there was a correlation with long-term facial function ≥1 year. All had normal preoperative facial function.
The EMG patterns were classified as follows: 1) an irritable pattern with repeated EMG responses elicited very easily and frequently by the slightest contact with the facial nerve, 2) a silent pattern with little or no EMG responses, 3) a stray pattern with persistent train responses up to 20 minutes despite temporary discontinuance of surgical manipulations, and 4) an ordinary pattern with EMG responses elicited by mechanical stimulation of the nerve but not very easily or frequently.
49 VS patients with at least 1-year follow-up (mean 18 months). Single center, single surgeon experience from April 1998–October 1999. NF2 status not reported.
HB grading system used.
|
III
|
An ordinary or irritable pattern predicted HB I–II in 85% and 95%, respectively. A silent pattern only predicted poor outcome HB III–VI in 73% (8/11).
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Fenton et al, 2002
|
Prospective study of 67 VS patients with normal preoperative facial function collected from 2 centers. 35 patients met study criteria. Looked at various other predictive factors, including tumor size and surgical approach. Also evaluated MIMAE relationship with long-term facial function (HB score) at 2-year follow-up.
MIMAE was obtained by using a constant current technique and a standard pulse duration of 100 seconds required to provoke a stimulus threshold event on an intact facial nerve medial to the tumor location (0.05–3.0 mA). Fisch dissector used.
Multi-institution, same senior surgeon, in 1994. NF2 patients excluded.
HB grading system used.
|
III
|
Immediate facial nerve function was the only independent predictor of facial nerve function at ≥1 year. MIMAE was not found to be predictive in their multivariable logistic regression model. MIMAE was significant in univariate analysis (odds ratio = 0.57).
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Yokoyama et al, 1999
|
Retrospective analysis of 66 VS patients. Evaluated intraoperative EFMR at the brainstem after tumor removal. Responses were measured as amplitudes (µV). This was correlated with facial nerve function at 18 months postsurgery.
Monopolar currents 0.5–0.6 mA with 100-ms pulse duration was used. Results were classified into 4 groups according to response levels.
Single center study. NF2 status not reported.
HB grading system used.
|
III
|
Amplitudes were not a good predictor of ultimate functional outcome (HB grade). They were a better predictor of time to recovery in patients who did recover; >150 µV: 3 months to recovery; 100–149 µV: 6 months; 50–99 µV: 9 months; <50 µV: 12 months (Mann–Whitney U test <0.05).
85% of their cohort with response amplitudes of ≥100 µV obtained long-term function of HB I. Function was unpredictable with levels <100 µV.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Mandpe et al, 1998
|
Prospective, nonconsecutive, 44 VS patients. Stimulation threshold at brainstem (volts) and amplitude proximal and distal to tumor resection at 0.2 V above threshold. Monopolar probe used. Correlated this with FN outcomes with ≥1-year follow-up.
Single center experience between 1994–1996. NF2 patients included (n = 3).
HB grading system used.
|
III
|
Combined threshold (≤0.1 V) and amplitude (≥200 µV) was superior to threshold alone at predicting early and late postoperative HB I–II FN function.
False positive rate was 12% (false positive = poor HB outcome despite favorable ICNM parameters).
Cannot reliably predict poor outcomes. 94% of patients with stimulation thresholds ≤0.1 V had HB I–II at 1 year. 89% of those with amplitude >200 µV had HB I–II function. Using combined parameters, 88% had HB I–II function.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Magliulo et al, 1998
|
Retrospective analysis of 34 VS patients. Compared 3 methods of intraoperative testing: 1) amplitude of train activity lasting 30 seconds (less or greater than 500 µV), 2) amplitude of response at brainstem at 0.05 mA (less than or greater than 500 µV), 3) ratio of stimulation intensity over the amplitude of evoked response.
Monopolar probe used. These 3 methods were analyzed in relation to 10-day and 1-year HB scores.
Two center experience between 1990–1994. NF status not reported.
HB grading system used.
|
III
|
The best method was the ratio of stimulation intensity over the amplitude of evoked response which predicted a good HB score in 90% of patients. The 10% that could not be predicted were the poor outcomes (P < .02).
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Zeitouni et al, 1997
|
Prospective study of 109 VS patients evaluating correlation between the minimum intraoperative stimulus threshold and 1-year facial function outcomes (HB score). Thresholds were measured using a constant current technique with a monopolar flush tip Prass probe at the brainstem. Minimum intensity used 0.05 mA.
Single center experience. NF2 status not reported.
HB grading system used.
|
III
|
Stimulus threshold was predictive of short- and good long-term facial function (P = .0032 and P = .048, respectively). Stimulus threshold of 0.05 mA or 0.1 mA predicted HB I–II in 87%. Could not predict long-term poor function.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Selesnick et al, 1996
|
Retrospective analysis of 49 VSs or CPA meningiomas patients. 14% of cohort had CPA meningioma. Stimulation of FN at root entry zone after tumor resection. Constant current method starting at 0.1 mA with 50 µsec pulse duration. Measured stimulation threshold at 0.1-mA increments. Monopolar stimulators were used (Kartush and Prass probe). Compared this to early and 1-year facial function (HB score.
Two center, single surgeon experience between 1991–1995. NF2 status not reported.
HB grading system used.
|
III
|
A stimulation of ≤0.2 mA predicted good facial nerve function (HB I or II) at 1 year (P < .01).
Could not predict poor function.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Taha et al, 1995
|
Retrospective analysis of 20 VS patients. Evaluated postresection proximal to distal ratios of amplitude of muscle action potential. They used the lowest intensity required to elicit a response (not a supramaximal stimulation). Compared this with short- and long-term (1 year) HB scores.
Mean follow-up was 18 months.
The nerve was stimulated at the brainstem and at the internal auditory meatus after tumor resection. Starting at 0.05 mA to a max of 1 mA. Monopolar stimulator probe was used with constant current at 4 pulses per second for 100 msec.
Single center experience between 1992–1993. NF status not reported.
HB grading system used.
|
III
|
A proximal to distal ratio of 2:3 was predictive of good long-term function (HB I). Although a ratio of 1:3 predicted poor function (HB IV or more), there were too few patients for adequate statistical analysis (n = 5), which was not performed.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Silverstein et al, 1994
|
Retrospective analysis of 44 VS patients. Minimum brainstem threshold response level (constant current, square wave pulse stimulation) in mA used to predict long-term facial function (HB score) at 1 year. Silverstein probe used (monopolar).
Single center experience between 1984–1991. NF status not reported.
HB grading system used.
|
III
|
Minimum threshold response of ≤0.1 mA predicted good long-term facial function (HB I) in 95% of patients at least 1 year after surgery. Could not predict poor outcomes.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
CMAP, compound muscle action potential; CPA, cerebellopontine angle; EFMR, evoked facial muscle response; EMG, electromyogram; FN, facial nerve; HB, House–Brackmann; MMax, maximum muscle response; MIMAE, medial to the tumor after excision; MST, minimum stimulus threshold; NF/NF2, neurofibromatosis; PPV, positive predictive value; SL, current stimulus; SMS, supramaximal stimulation.
Table 3C. Evidence table for question 3
|
Author/Year
|
Study Description
|
Data Class
|
Conclusion
|
|
Carlson et al, 2012
|
Retrospective review of 11 VS patients with no measured electrical response at the end of VS surgery and anatomically intact nerves. These patients were followed for >1 year to see if “no response” parameter could predict poor facial nerve function. Monopolar Prass probe was used.
Single center, experience from 2000–2010, mean follow-up of 81.8 months. NF2 patients excluded.
HB grading system used.
|
III
|
“No electrical response” was unable to predict poor (HB IV–VI) long-term facial function at >1 year. Only 36% (4/11) had a poor outcome. 18% (2/11) had a HB II recovery which would be superior to current facial reinnervation procedure results.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Amano et al, 2011
|
Retrospective review of continual stimulation evoked facial nerve EMG. Calculated an amplitude preservation ratio (%) during and after tumor resection and evaluated whether this could predict long-term facial function at ≥1 year. The facial nerve was electrically stimulated with monopolar current 0.1–3 mA at 1 Hz and CMAP continuously monitored. Free running spontaneous EMG, as well as evoked facial EMG were used. The stimulation was via monopolar probe placed at the nerve’s root exit zone at the brainstem.
The amplitude preservation ratio (%) = {last M-max(µV)/Control M-max (µV)} × 100
Control M-max = maximum CMAP amplitude at start of surgery
Total patient sample of 232 consecutive VS patients excluding 16 patients with preoperative facial weakness, prior surgery, or radiation (n = 216).
Single center experience from 2005–2008. NF2 patients excluded.
HB grading system used.
|
III
|
Concluded that continual stimulation evoked facial nerve EMG could be used to determine if tumor resection should continue. An amplitude preservation ratio >50% or last amplitude measured (Mmax) >1000 µV was predictive of good facial function. A ratio >50% had 95% HB I or II. Unclear what >1000 µV predicted. A discrepancy with µV criteria is that they reached this cutoff with several tumors with a large amount of remnant; thus continued to operate.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Prell et al, 2007
|
Retrospective review of whether A-train duration measured from free running EMG could predict facial nerve function at 1 year postoperatively. A-train activity is defined as a close succession of at least 4 geometrically
similar, mono- to triphasic discharges from baseline
with an amplitude of at least double background noise. The sequence of at least four elements is required to build a frequency of 100–200 Hz, which must be steady over the
course of any given A train. Train time is reported in seconds.
40 VS patients with a minimum of 1-year follow-up. 26 had normal preoperative facial function and 14 did not.
Single center experience from 1994–2003. NF2 status not reported.
HB grading system used.
|
III
|
For the patients with normal preoperative function and A-train time <10 seconds, 81% had normal function at 1 year. For the entire cohort, an A-train time of >10 seconds predicted a HB II–VI (everyone but normal HB I) in 81% at 1 year of follow-up. 5 of 40 (13%) with prolonged A-Train times still became HB I. Sensitivity
was 57.1% and specificity 81% for the 10-second threshold.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Nakao et al, 2002
|
Prospective analysis of EMG “pattern” (irritable, silent, stray, or ordinary) during the critical portion of tumor removal to see if there was a correlation with long-term facial function ≥1 year. All had normal preoperative facial function.
The EMG patterns were classified as follows: 1) an irritable pattern with repeated EMG responses elicited very easily and frequently by the slightest contact with the facial nerve, 2) a silent pattern with little or no EMG responses, 3) a stray pattern with persistent train responses up to 20 minutes despite temporary discontinuance of surgical manipulations, and 4) an ordinary pattern with EMG responses elicited by mechanical stimulation of the nerve but not very easily or frequently.
49 VS patients with at least 1-year follow-up (mean 18 months).
Single center, single surgeon experience from April 1998–October 1999. NF2 status not reported.
HB grading system used.
|
III
|
An ordinary or irritable pattern predicted HB I–II in 85% and 95%, respectively. A silent pattern only predicted poor outcome HB III–VI in 73% (8/11).
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
CMAP, compound muscle action potential; EMG, electromyogram; FN, facial nerve; HB, House–Brackmann; MMax, maximum muscle response; NF2, neurofibromatosis.
Table 4A. Evidence table for question 4
|
Author/Year
|
Study Description
|
Data Class
|
Conclusion
|
|
Piccirillo et al, 2008
|
Retrospective analysis of hearing preservation (modified Sanna class A–B) in patients undergoing surgical resection of VSs with or without use of ICNM at a single center from 1998–2005 by a single surgeon.
99 cases of tumor <1.5 cm, NF2 excluded, and with Sanna class A–B (AAO-HNS class A) preoperative hearing.
Fast-ABR (5 sec) and direct cochlear nerve action potentials were obtained. These were measured by placing an electrode directly on the cochlear nerve.
There were 2 groups. Group 1 consisted of patients with either DENM or auditory brain stem response. Group 2 included patients with no intraoperative monitoring.
|
III
|
Across all surgeries, those with ICNM (ABR, DENM, or both) did not have a statistically greater chance of hearing preservation, though it trended in this direction (26.7% vs 20.8%, P = .79).
Surgical approach, either MCF or RS/RL, did not have a significant effect.
The only statistically significant parameter was prognostic value to the presence of continued, appropriate stimulation with ICNM. Meaning, if there was a positive response at the end of the case, you were statistically likely to have preserved hearing (P < .01, Fisher exact test).
Author conclusions:
ICNM does not help preserve hearing, but it may have prognostic value.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Nedzelski et al, 1994
|
Retrospective analysis of hearing preservation after VS resection via suboccipital approach at a single center between 1975–1993. Number of surgeons involved not specified.
80 cases were evaluated in which hearing preservation was attempted. The measurement/prognostic value of cochlear CAPs vs no monitoring was evaluated. 56 patients had CAP measurements during the case and the remainder did not (the remainder also including 4 patients who had unreliable CAP measurements from the start). CAP was obtained by placing a silver ball electrode directly on the promontory via myringotomy. Threshold shifts were calculated as the difference between threshold measurements at the beginning and end of surgery.
All patients had tumors ≤1.5 cm and SRT <50 and WRS >60%.
Postoperative hearing was followed for 1 year after surgery.
|
III
|
Significantly better rates of hearing preservation were seen with patients in whom CAP was measured intraoperatively (38% vs 15%, P = .02). Results were not correlated to tumor size.
In cases where CAP was present and unchanged, 53% of patients had serviceable hearing. One patient with complete loss of CAP had serviceable hearing.
CAP threshold shifts of ≤20 dB predicted successful serviceable hearing preservation postoperatively in 71% of cases (P = .001). >20 dB shift predicted loss of serviceable hearing (P < .003).
Author conclusions:
Intraoperative CAP monitoring can be useful for hearing preservation attempts.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Harper et al, 1992
|
Retrospective comparison of hearing preservation rates using ABR vs no monitoring during VS resection via suboccipital approach at a single center between 1986–1991 by multiple surgeons.
There were 90 consecutive patients who underwent a hearing preservation attempt with use of ABR. A control group of 90 patients who were matched for age, tumor size, and preoperative hearing were included.
All patients had preoperative PTA <65 dB and WRS of >40%. Frequencies used to calculate PTA not specified.
Postoperative hearing assessed at 3 months. “Preserved” hearing = PTA <65 dB; “useful” preserved hearing = WRS >40%.
|
III
|
When comparing the groups across all tumor sizes, the ABR group trended towards better hearing preservation and better useful hearing preservation, but the differences were not statistically significant.
When comparing the groups for tumors <1.1 cm, the ABR group had better hearing preservation (79% vs 42%) and better rates of useful preservation (47% vs 21%). This was the only size category in which there was a statistically significant difference (P < .05). If ABR waves I and V preserved, then 67% chance of useful hearing.
Author conclusions:
ABR is better than no monitoring, particularly when tumors are <1.1 cm.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Slavit et al, 1991
|
Retrospective analysis of hearing preservation after VS resection via posterior fossa approach at a single center between 1986–1989. Comparison included use of cochlear nerve monitoring by ABR vs no monitoring. All procedures performed by same surgical team.
60 patients with some preoperative hearing and use of intraoperative ABR were matched with 60 patients with no ABR on the basis of tumor size (within 2 mm), preoperative PTA (at 500, 1000, and 2000 Hz), word discrimination scores, and year of operation
Tumor size classified as “small” when <2 cm, medium when 2–4 cm, and large when >4 cm.
Follow up included 1 week and 3-month postoperative audiogram.
Preservation = anything measurable. Useful preservation = PTA <60 dB, WRS >40%
|
III
|
No tumor >3 cm had hearing preserved.
Rates of preservation trended towards higher with ABR, but this was not statistically significant (30% with ABR, 20% without ABR). P values not provided.
The difference became more pronounced when focusing on tumors <1 cm (82% preservation with ABR, 36% without) but still not statistically significant. P values not provided.
The difference for the preservation of useful hearing was also not significantly different. The level of preoperative hearing did not seem to matter in terms of rates of preservation in the 2 groups.
Author conclusions:
Definite trend favoring the monitored group to improved rates of hearing preservation, but nothing statistically significant.
ABR could not always reliably tell when the cochlear nerve was cut.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
|
Kveton, 1990
|
Retrospective analysis of hearing preservation after VS resection via suboccipital-transmeatal approach by a single surgeon at a single center between 1987–1989. A comparison was performed between cases with intraoperative ABR to ones in which no monitoring was used.
16 cases were evaluated in which there was a hearing preservation attempt. Nine patients had intraoperative ABR, and 7 had no form of monitoring. All tumors were ≤2 cm.
The cochlear nerve was anatomically intact in all cases.
All patients had preoperative AAO-HNS class B hearing or better. Pre- and postoperative SRT/WRS are listed for comparison. 50/50 criterion used to define “serviceable” hearing. Postoperative audiograms measured at variable intervals (from 2 months to 1 year postoperatively).
|
III
|
No significant difference in preoperative tumor size or postoperative hearing outcome between the monitored and unmonitored groups.
Postoperative serviceable hearing preservation was greater in the non-ABR than monitored group (57% vs 44%, not significant).
No formal analysis of age or tumor size or preoperative hearing status as potential confounders.
Author conclusions:
ABR not very helpful.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; ABR, auditory brainstem response; DENM, direct eighth nerve monitoring; ICNM, intracranial cochlear nerve monitoring; MCF, middle cranial fossa; PTA, pure tone average; RS/RL, retrosigmoid-retrolabyrinthine; SRT, speech recognition threshold; VS, vestibular schwannoma; WRS, word recognition score.
Table 4B. Evidence table for question 5
|
Author/Year
|
Study Description
|
Data Class
|
Conclusion
|
|
Danner et al, 2004
|
Retrospective comparison of hearing preservation rates using either ABR or DENM following VS resection via retrosigmoid approach by a single surgeon between 1992–2002.
66 patients were included in the study for comparison of DENM and ABR. 22 patients were monitored with ABR and 44 with DENM.
Patients with a tumor >2.5 cm were excluded from the analysis as none had hearing preservation achieved.
All patients had preoperative AAO-HNS class B hearing or better (SRT <50 dB, WRS >50%).
Unclear when postoperative audiogram was performed.
|
III
|
Analysis of all cases, regardless of tumor size (0–2.5 cm), found the use of DENM had a statistically greater chance of hearing preservation than ABR (P = .03). However, the differences between tumor sizes in the 2 groups (ABR and DENM) is not well addressed.
Hearing preservation
analysis by size subcategories (<1 cm, 1–1.5 cm, 1.5–2 cm, and 2–2.5 cm), found no statistical difference in any group between DENM and ABR.
DENM had improved rates of hearing preservation that trended towards significance with an exception of the 2–2.5 cm group where rates were equal.
The type of eighth nerve monitoring did not affect postoperative facial nerve preservation or CSF leak rates.
Author conclusions:
DENM gives better rates of hearing preservation in tumors <2 cm. DENM is shown to have a statistically significant advantage when comparing all study patients with tumors <2.5 cm.
Classification of evidence on prognosis class III. Did not blind outcome measure. No validation in an independent “test set” of patients.
|
AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; ABR, auditory brainstem response; CSF, cerebrospinal fluid; DENM, direct eighth nerve monitoring; SRT, speech recognition threshold; VS, vestibular schwannoma; WRS, word recognition score.
References
1. Mass SC, Wiet RJ, Dinces E. Complications of the translabyrinthine approach for the removal of acoustic neuromas. Arch Otolaryngol Head Neck Surg 1999;125(7):801-804.
2. Sughrue ME, Yang I, Rutkowski MJ, Aranda D, Parsa AT. Preservation of facial nerve function after resection of vestibular schwannoma. Br J Neurosurg 2010;24(6):666-671.
3. Delgado TE, Bucheit WA, Rosenholtz HR, Chrissian S. Intraoperative monitoring of facila muscle evoked responses obtained by intracranial stimulation of the facila nerve: a more accurate technique for facila nerve dissection. Neurosurgery 1979;4(5):418-421.
4. National Institutes of Health Consensus Development Conference Statement on Acoustic Neuroma, December 11-13, 1991. The Consensus Development Panel. Arch Neurol 1994;51(2):201-207.
5. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg 1985;93(2):146-147.
6. Ross BG, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngol Head Neck Surg 1996;114(3):380-386.
7. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg 1995;113(3):179-180.
8. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol 1988;97(1):55-66.
9. Amano M, Kohno M, Nagata O, Taniguchi M, Sora S, Sato H. Intraoperative continuous monitoring of evoked facial nerve electromyograms in acoustic neuroma surgery. Acta Neurochir (Wien) 2011;153(5):1059-1067.
10. Arnoldner C, Mick P, Pirouzmand F, et al. Facial nerve prognostication in vestibular schwannoma surgery: the concept of percent maximum and its predictability. Laryngoscope 2013;123(10):2533-2538.
11. Carlson ML, Van Abel KM, Schmitt WR, Driscoll CL, Neff BA, Link MJ. The anatomically intact but electrically unresponsive facial nerve in vestibular schwannoma surgery. Neurosurgery 2012;71(6):1125-1130.
12. Fenton JE, Chin RY, Fagan PA, Sterkers O, Sterkers JM. Predictive factors of long-term facial nerve function after vestibular schwannoma surgery. Otol Neurotol 2002;23(3):388-392.
13. Fenton JE, Chin RY, Shirazi A, Fagan PA. Prediction of postoperative facial nerve function in acoustic neuroma surgery. Clin Otolaryngol Allied Sci 1999;24(6):483-486.
14. Palasti S, Kwartler J. Temporal bone histopathology: residents' quiz. Am J Otolaryngol 1991;12(5):299-301.
15. Lalwani AK, Butt FY, Jackler RK, Pitts LH, Yingling CD. Facial nerve outcome after acoustic neuroma surgery: a study from the era of cranial nerve monitoring. Otolaryngol Head Neck Surg 1994;111(5):561-570.
16. Lenarz T, Ernst A. Intraoperative facial nerve monitoring in the surgery of cerebellopontine angle tumors: improved preservation of nerve function. ORL J Otorhinolaryngol Relat Spec 1994;56(1):31-35.
17. Magliulo G, Zardo F. Facial nerve function after cerebellopontine angle surgery and prognostic value of intraoperative facial nerve monitoring: a critical evaluation. Am J Otolaryngol 1998;19(2):102-106.
18. Mandpe AH, Mikulec A, Jackler RK, Pitts LH, Yingling CD. Comparison of response amplitude versus stimulation threshold in predicting early postoperative facial nerve function after acoustic neuroma resection. Am J Otol 1998;19(1):112-117.
19. Marin P, Pouliot D, Fradet G. Facial nerve outcome with a peroperative stimulation threshold under 0.05 mA. Laryngoscope 2011;121(11):2295-2298.
20. Nakao Y, Piccirillo E, Falcioni M, et al. Prediction of facial nerve outcome using electromyographic responses in acoustic neuroma surgery. Otol Neurotol 2002;23(1):93-95.
21. Neff BA, Ting J, Dickinson SL, Welling DB. Facial nerve monitoring parameters as a predictor of postoperative facial nerve outcomes after vestibular schwannoma resection. Otol Neurotol 2005;26(4):728-732.
22. Prell J, Rampp S, Romstock J, Fahlbusch R, Strauss C. Train time as a quantitative electromyographic parameter for facial nerve function in patients undergoing surgery for vestibular schwannoma. J Neurosurg 2007;106(5):826-832.
23. Schmitt WR, Daube JR, Carlson ML, et al. Use of supramaximal stimulation to predict facial nerve outcomes following vestibular schwannoma microsurgery: results from a decade of experience. J Neurosurg 2013;118(1):206-212.
24. Selesnick SH, Carew JF, Victor JD, Heise CW, Levine J. Predictive value of facial nerve electrophysiologic stimulation thresholds in cerebellopontine-angle surgery. Laryngoscope 1996;106(5 pt 1):633-638.
25. Silverstein H, Rosenberg SI, Flanzer J, Seidman MD. Intraoperative facial nerve monitoring in acoustic neuroma surgery. Am J Otol 1993;14(6):524-532.
26. Silverstein H, Willcox TO, Jr., Rosenberg SI, Seidman MD. Prediction of facial nerve function following acoustic neuroma resection using intraoperative facial nerve stimulation. Laryngoscope 1994;104(5 pt 1):539-544.
27. Taha JM, Tew JM, Jr., Keith RW. Proximal-to-distal facial amplitude ratios as predictors of facial nerve function after acoustic neuroma excision. J Neurosurg 1995;83(6):994-998.
28. Zeitouni AG, Hammerschlag PE, Cohen NL. Prognostic significance of intraoperative facial nerve stimulus thresholds. Am J Otol 1997;18(4):494-497.
29. Yokoyama T, Nishizawa S, Sugiyama K, Yokota N, Ohta S, Uemura K. Intraoperative evoked facial muscle responses and recovery process of the facial nerve in acoustic neuroma surgery. Br J Neurosurg 1999;13(6):570-575.
30. Kwartler JA, Luxford WM, Atkins J, Shelton C. Facial nerve monitoring in acoustic tumor surgery. Otolaryngol Head Neck Surg 1991;104(6):814-817.
31. Piccirillo E, Hiraumi H, Hamada M, Russo A, De Stefano A, Sanna M. Intraoperative cochlear nerve monitoring in vestibular schwannoma surgery--does it really affect hearing outcome? Audiol Neurootol 2008;13(1):58-64.
32. Danner C, Mastrodimos B, Cueva RA. A comparison of direct eighth nerve monitoring and auditory brainstem response in hearing preservation surgery for vestibular schwannoma. Otol Neurotol 2004;25(5):826-832.
33. Nedzelski JM, Chiong CM, Cashman MZ, Stanton SG, Rowed DW. Hearing preservation in acoustic neuroma surgery: value of monitoring cochlear nerve action potentials. Otolaryngol Head Neck Surg 1994;111(6):703-709.
34. Kveton JF. The efficacy of brainstem auditory evoked potentials in acoustic tumor surgery. Laryngoscope. Nov 1990;100(11):1171-1173.
35. Harper CM, Harner SG, Slavit DH, et al. Effect of BAEP monitoring on hearing preservation during acoustic neuroma resection. Neurology 1992;42(8):1551-1553.
36. Slavit DH, Harner SG, Harper CM, Jr., Beatty CW. Auditory monitoring during acoustic neuroma removal. Arch Otolaryngol Head Neck Surg 1991;117(10):1153-1157.
37. Kemink JL, LaRouere MJ, Kileny PR, Telian SA, Hoff JT. Hearing preservation following suboccipital removal of acoustic neuromas. Laryngoscope 1990;100(6):597-602.
© Congress of Neurological Surgeons
Source: Neurosurgery, February 2018