Guidelines on the Management of Patients with Vestibular Schwannoma
5. The Role of Imaging in the Diagnosis and Management of Patients with Vestibular Schwannomas
download pdf Neurosurgery, 2017
Sponsored by: Congress of Neurological Surgeons (CNS) and the AANS/CNS Tumor Section
Endorsed by: Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)
Authors:
Ian F. Dunn, MD1, Wenya Linda Bi, MD, PhD1, Srinivasan Mukundan, MD, PhD2, Bradley N. Delman, MD3, Jonathan Parish, MD4, Tyler Atkins, MD5, Anthony L. Asher, MD4, Jeffrey J. Olson, MD5
1. Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
2. Division of Neuroradiology, Brigham and Women’s Hospital, Boston, Massachusetts, USA
3. Department of Radiology (Neuroradiology), Icahn School of Medicine at Mount Sinai, New York, New York, USA
4. Carolinas Medical Center, Charlotte, North Carolina, USA
5. Carolina Neurosurgery & Spine Associates, Charlotte, North Carolina, USA
6. Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
Correspondence:
Ian F. Dunn, MD
Department of Neurosurgery
Brigham and Women’s Hospital
Boston, Massachusetts, USA
IDUNN@PARTNERS.ORG
Keywords: Acoustic neuroma, advanced imaging, cystic, growth rate, facial nerve, MRI, vestibular schwannoma
No part of this manuscript has been published or submitted for publication elsewhere.
Abbreviations
AN: Acoustic neuroma
AAO-HNS: American Academy of Otolaryngology-Head and Neck Surgery
CISS: Constructive interference in steady state
CPA: Cerebellopontine angle
CSF: Cerebrospinal fluid
CT: Computed tomography
DTI: Diffusion tensor imaging
DTT: Diffusion tensor tractography
DWI: Diffusion weighted imaging
EMG: Electromyography
FFE: Fast-field echo
FIESTA: Fast imaging employing steady-state acquisition
FLAIR: Fluid attenuated inversion recovery
FN: Facial nerve
FSE: Fast spin echo
GRE: Gradient echo
GTR: Gross total resection
HB: House–Brackmann
HP: Hearing preservation
IAC: Internal auditory canal
MF: Middle fossa
MPRAGE: Magnetization prepared rapid acquisition gradient echo
MRI: Magnetic resonance imaging
NEA: Nonenhancing area
NF2: Neurofibromatosis type 2
NGR: No growth rate
NTR: Near-total resection
PRESTO: Principles of echo-shifting with a train of observations
RS: Retrosigmoid
SIMCAST: Segment-interleaved motion-compensated acquisition in steady state
SRS: Stereotactic radiosurgery
SSHL: Sudden sensorineural hearing loss
STR: Subtotal resection
TSE: Turbo spin echo
TL: Translabyrinthine
VDE: Velocity of diametric expansion
VDT: Volume doubling time
VS: Vestibular schwannoma
VSTR: Vestibular schwannoma tumor remnant
Abstract
Question 1
What sequences should be obtained on MRI to evaluate vestibular schwannomas before and after surgery?
Target Population
Adults with vestibular schwannomas
Recommendation
Initial Preoperative Evaluation
Level 3: Imaging used to detect vestibular schwannomas should use high-resolution T2-weighted and contrast-enhanced T1-weighted MRI.
Level 3: Standard T1, T2, FLAIR, and DWI MR sequences obtained in axial, coronal, and sagittal plane may be used for detection of vestibular schwannomas.
Preoperative Surveillance
Level 3: Preoperative surveillance for growth of a vestibular schwannoma should be followed with either contrast-enhanced 3D T1 MPRAGE or high-resolution T2 (including CISS or FIESTA sequences) MR imaging.
Postoperative Evaluation
Level 2: Postoperative evaluation should be performed with post-contrast 3D T1 MPRAGE, with nodular enhancement considered suspicious for recurrence.
Question 2
Is there a role for advanced imaging for facial nerve detection preoperatively (eg, CISS/FIESTA or DTI imaging)?
Target Population
Adults with proven or suspected vestibular schwannomas by imaging
Recommendation
Level 3: T2-weighted MRI may be used to augment visualization of the facial nerve course as part of preoperative evaluation.
Question 3
What is the expected growth rate of vestibular schwannomas on MRI, and how often should they be imaged if a “watch and wait” philosophy is pursued?
Target Population
Adults with suspected vestibular schwannomas by imaging
Recommendation
Level 3: MRIs should be obtained annually for 5 years, with interval lengthening thereafter with tumor stability.
Question 4
Do cystic vestibular schwannomas behave differently than their solid counterparts?
Target Population
Adults with vestibular schwannomas with cystic components
Recommendation
Level 3: Adults with cystic vestibular schwannomas should be counseled that their tumors may more often be associated with rapid growth, lower rates of complete resection, and facial nerve outcomes that may be inferior in the immediate postoperative period but similar to non-cystic schwannomas over time.
Question 5
Should the extent of lateral internal auditory canal (IAC) involvement be considered by treating physicians?
Target Population
Adult patients with a vestibular schwannomas
Recommendation
Level 3: The degree of lateral IAC involvement by tumor adversely affects facial nerve and hearing outcomes and should be emphasized when interpreting imaging for preoperative planning.
Question 6
How should patients with neurofibromatosis type 2 (NF2) and vestibular schwannoma be imaged and over what follow-up period?
Target Population
Adult patients with neurofibromatosis type 2 and vestibular schwannomas
Recommendation
Level 3: In general, vestibular schwannomas associated with NF2 should be imaged (similar to sporadic schwannomas) with the following caveats:
1. More frequent imaging may be adopted in NF2 patients because of a more variable growth rate for vestibular schwannomas, and annual imaging may ensue once the growth rate is established.
2. In NF2 patients with bilateral vestibular schwannomas, growth rate of a vestibular schwannoma may increase after resection of the contralateral tumor, and therefore, more frequent imaging may be indicated, based on the non-operated tumor’s historical rate of growth.
3. Careful consideration should be given to whether contrast is necessary in follow-up studies or if high-resolution T2 (including CISS or FIESTA-type sequences) MRI may adequately characterize changes in lesion size instead.
Question 7
How long should vestibular schwannomas be imaged after surgery, including after gross total, near total, and subtotal resection?
Target population
Adult patients with vestibular schwannomas followed after surgery
Recommendation
Level 3: For patients receiving gross total resection, a postoperative MRI may be considered to document the surgical impression and may occur as late as 1 year after surgery. For patients not receiving gross total resection, more frequent surveillance scans are suggested; annual MRI scans may be reasonable for 5 years. Imaging follow-up should be adjusted accordingly for continued surveillance if any change in nodular enhancement is demonstrated.
Introduction
Vestibular schwannomas (VSs) are the most common intracranial nerve sheath tumor, arising from the vestibular division of the vestibulocochlear (VIIIth) nerve. VSs are usually sporadic in origin but may also arise in the context of neurofibromatosis type 2 (NF2) and present with a familiar constellation of symptoms including but not limited to, hearing loss, dizziness, vertigo, and, in larger tumors, symptoms related to cerebellar or brainstem compression. Management of VSs has classically included watchful waiting with surveillance imaging, treatment with microsurgical resection, or radiotherapy in one of several forms. Symptoms, tumor size, and specific patient and surgeon characteristics and preferences influence the choice of which treatment is adopted. While these broad themes are considered from center to center, considerable variation in treatment patterns exists among practitioners.
The detection of VSs is usually done using MRI, which provides unparalleled radiographic analysis and confers opportunities to establish an understanding of the natural history in cases where conservative management is pursued. The growth rates of surgical remnants may also be followed reliably using MRI. Moreover, specific details of tumor makeup (including the extent of intracanalicular involvement and the presence of cystic components, among others) can be discerned, and novel MRI sequences may also be applied to their radiographic assessment.
The following review was performed to provide a set of evidence-based recommendations for the use of imaging in the management of patients with VSs.
Objectives
This article aims to critically analyze the primary literature regarding the role of imaging in the management of VSs based on the following questions:
1. What sequences should be obtained on MRI to evaluate vestibular schwannomas before and after surgery?
2. Is there a role for advanced imaging for facial nerve detection preoperatively? (eg, CISS/FIESTA or DTI imaging)
3. What is the expected growth rate of vestibular schwannomas on MRI, and how often should they be imaged if a “watch and wait” philosophy is pursued?
4. Do cystic vestibular schwannomas behave differently than their solid counterparts?
5. Should the extent of lateral internal auditory canal (IAC) involvement be considered by treating physicians?
6. How should patients with neurofibromatosis type 2 (NF2) and vestibular schwannomas be imaged and over what follow-up period?
7. How long should vestibular schwannomas be imaged after surgery, including after gross total, near total, and subtotal resection?
Methods
Process Overview
The evidence-based clinical practice guideline taskforce members and the Joint Tumor Section of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) conducted a systematic review of the literature relevant to the management of VSs. Additional details of the systematic review are provided below and within the introduction and methodology chapter of the guideline (here).
During the development process, the panel participated in a series of conference calls and meetings. Multiple iterations of written review were conducted by the individuals of the panel and the AANS/CNS Joint Guidelines Committee prior to approval. A list of members of the guideline task force can be found in the guideline introductory publication.
Study Selection and Eligibility Criteria
A total of 2070 citations were manually reviewed. Two independent reviewers evaluated and abstracted full-text data for each article. Citations focused on the imaging of VSs in adult patients largely in the MRI era (January 1, 1990 to December 31, 2014), published in English, were considered.
- Investigated patients suspected of having VSs
- Patients ≥18 years of age
- Was of humans
- Published between January 1, 1946 and December 31, 2014
- Quantitatively presented results
- Was not an in vitro study (for novel molecular markers, in vitro studies were included on patient samples)
- Was not a biomechanical study
- Was not performed on cadavers
- Was published in English
- Was not a, meeting abstract, editorial, letter, or commentary
- Studies may include mixed pathology, however the data pertaining to acoustic neuromas (ANs)/VSs was abstractable from the paper.
- >5 patients or patient samples
Systematic reviews, guidelines, or meta-analyses conducted by other authors were not included in this guideline creation. These documents were developed using different inclusion criteria than those specified in this guideline. Therefore, they may have included studies that do not meet the inclusion criteria stated above.
Search Strategies
The task force collaborated with a medical librarian to search for articles published between January 1, 1990 and December 31, 2014. The following electronic databases were searched: PubMed and Cochrane Central. Strategies for searching electronic databases were constructed by the evidence-based clinical practice guideline taskforce members and the medical librarian using previously published search strategies to identify relevant studies (Figure 1; Table 1). The guideline committee also examined lists of included and excluded studies for errors and omissions.
Data Collection Process and Assessment of Bias
Abstracts that met the inclusion criteria were retrieved in full text form and evaluated for confirmation that they met criteria as suggested by prior abstract review. The information was then used for construction of the evidence tables.
The possibility of systematic bias in results was addressed by first stratifying the evidence based on the class of evidence quality, which highlights the limitations in this literature. Given the dearth of evidence for many of these questions, formal methods for studying publication bias, such as funnel plots were not feasible.
In addition, one obvious bias inherent to these studies is selection bias. For a patient to be in an imaging study, that patient, by definition, underwent imaging for a clinical reason, which may bias results toward larger and possibly more aggressive tumors than would be seen in a cohort of all VSs. However, it is important to note that this bias is uniform across all studies of this type. Therefore, while individual practitioners may have skewed results by differences in case selection, there is no clear mechanism by which these biases are systematically distributed.
Classification System and Recommendation Formulation
The concept of linking evidence to recommendations has been further formalized by the American Medical Association (AMA) and many specialty societies, including the AANS, CNS, and the American Academy of Neurology (AAN). This formalization involves the designation of specific relationships between the strength of evidence and the strength of recommendations to avoid ambiguity. In the paradigm for diagnostic maneuvers, evidence is classified into that which is derived from well-designed studies of a diverse population using a “gold standard” reference test in a blinded evaluation, or class I evidence. Class I evidence is used to support recommendations of the strongest type, defined as level 1 recommendations, indicating a high degree of clinical certainty. Well-designed studies of a restricted population using a “gold standard” reference test in a blinded evaluation provide class II evidence. These are used to support recommendations defined as level 2 reflecting a moderate degree of clinical certainty. Other sources of information, including expert opinions or studies that do not delineate sensitivity, specificity, positive and negative predictive values, and likelihood ratios, are considered class III evidence and SUPPORT Level 3 recommendations, reflecting unclear clinical certainty. A summary of these categories of evidence can be viewed at here
Results
Question 1
What sequences should be obtained on MRI to evaluate vestibular schwannomas before and after surgery?
Target population
Adults with vestibular schwannomas
Recommendations:
Initial Preoperative Evaluation
Level 3: Imaging used to detect vestibular schwannomas should use high-resolution T2-weighted and contrast-enhanced T1-weighted MRI.
Level 3: Standard T1, T2, FLAIR, and DWI MR sequences obtained in axial, coronal, and sagittal plane may be used for detection of vestibular schwannomas.
Preoperative Surveillance
Level 3: Preoperative surveillance for growth of a vestibular schwannoma should be followed with either contrast-enhanced 3D T1 MPRAGE or high-resolution T2 (including CISS or FIESTA sequences) MR imaging.
Postoperative Evaluation
Level 2: Post-operative evaluation should be performed with post-contrast 3D T1 MPRAGE, with nodular enhancement considered suspicious for recurrence.
Study Selection
Seventy full text articles published between 1990 and 2015 were initially reviewed. Of these 70 articles, 16 papers published before 1995 were excluded because of limited utility (eg, used older technology, retrospective or descriptive nature of the papers). Subsequently, articles were then divided into: 1) initial diagnosis (12 papers; Table 2) and 2) postoperative surveillance (10 papers; Table 3).
Risk of Bias and Limitations
Many papers were retrospective and nearly all papers were limited to individuals presenting with symptoms suggestive of VSs or other cerebellopontine angle mass lesion. In addition, there was variability in many technical parameters involving MRI. Fundamental elements including imaging slice thicknesses, acquisition plane, use of specific contrast agents, MRI field strength (3.0, 1.5, and 0.2 T were used) and method for image review (film, picture archiving and communication system, and 3D workstation) varied throughout this set of manuscripts, thereby potentially compromising both qualitative and quantitative accuracy.
Study Characteristics and Results of Studies
Initial Evaluation
Numerous studies over the last 20 years have used high-resolution MRI techniques to evaluate the presence of VSs in patients. MRI is superior to computed tomography (CT) for evaluation of VSs,1,2 although CT provides valuable information on bony anatomy for the surgeon. Initially, studies included a variety of high-resolution 2D T1 and T2 techniques,1,3,4 but quickly progressed to 3D techniques. The 2 most commonly used techniques are 3D T2 CISS and 3D T1 MPRAGE postcontrast imaging. Stuckey et al5 reported high sensitivity (94-100%) and specificity (94-98%) for the ability of CISS to detect tumor. Hermens et al6 demonstrated a high kappa for both intra- (0.93–1) and interobserver (0.83–0.84) reproducibility of the sensitivity (89–94%) and specificity (94–97%) of results.
Standard T1, T2, DWI, and FLAIR imaging also provides high sensitivity (96–100%) and specificity (88–93%).7,8 The role of FLAIR imaging as an adjunct technique has been raised by many studies as a means of identifying abnormal signal in the affected side in the setting of VSs.9 None of these studies demonstrated that this is an essential component of diagnosis.
Similarly, one study raises the use of T2 sequences as a means of identifying microhemorrhage as an adjunctive sign for the presence of a VS rather than other cerebellopontine angle (CPA) pathologies.10 However, this is adjunctive and not of primary diagnostic concern. The 3D T2 techniques may also help play a role in identifying the nerve of origin of masses and the extent of involvement of the IAC, but are not of primary diagnostic concern.11
Preoperative Surveillance
High-resolution T2 CISS imaging demonstrates equal characterization of tumor size as postcontrast T1-weighted imaging.11,12 However, T2 CISS imaging does not appear to supplant postcontrast T1-weighted imaging for identifying regions of necrosis and understanding internal tumor architecture.13 Of note, these apparent changes in internal architecture suggestive of necrosis may sometimes reflect artifacts produced by different temporal phases of imaging after contrast administration.14
At the time of publication, the evolving concerns around gadolinium retention within the brain and nephrogenic systemic sclerosis raise the consideration of avoiding contrast altogether if the overarching goal of routine surveillance is to identify lesion growth.15–17 If there is significant change in tumor size or clinical presentation, the patient could obtain postcontrast imaging at that time.
Posttreatment Surveillance
Several studies demonstrate that almost all postsurgical beds initially demonstrate enhancement that is typically thin and non-nodular.18–21 Such linear enhancement may persist for several years, but typically diminishes in avidity over time.22 In addition, the use of fibrin and muscular tissue or fat grafts for reconstruction may produce a nodular enhancement as early as 3 days after surgery and should prompt postoperative imaging within the first 2 days23,24 and include fat suppression sequences. In comparison, the development of nodular enhancement is highly correlated with tumor recurrence.18–20,25
The precise algorithm for surveillance is not clear, but most reports suggested that there might be a role for postoperative imaging at 1 and 5 years. Interval surveillance regimens varied, but annual imaging as remote as 10 years postoperatively were used in some studies. In comparison, after the initial diagnosis of a VS, a MRI at 6 months to identify tumors likely to grow followed by annual MRIs for 5 years is recommended.
Synthesis of Results
Class II evidence demonstrates that excellent preoperative identification of VSs can be achieved using 3D T2 CISS or postcontrast 3D T1 MPRAGE MRI. When these methods are unavailable, high-quality results may be obtained using T1, T2, FLAIR, and DWI images in the three main orthogonal planes. The relative equivalence in evaluating lesion size between T2 CISS and postcontrast 3D T1 MPRAGE imaging suggests that non-contrast imaging may be useful for monitoring lesion size. The development of nodular enhancement on postcontrast imaging is the hallmark of recurrent lesions.
Question 2
Is there a role for advanced imaging for facial nerve detection preoperatively (eg, CISS/FIESTA or DTI imaging)?
Target population
Adults with proven or suspected vestibular schwannomas by imaging
Recommendation
Level 3: T2-weighted MRI may be used to augment visualization of the facial nerve course as part of preoperative evaluation.
Study Selection
Twenty-two full-text articles published between 1990 and 2015 were reviewed, and 13 articles were included in this discussion (Table 4). Nine publications were excluded that did not address the role of advance imaging modalities or the detection of FN course.
Risk of Bias and Limitations
Many studies in this analysis were retrospective and therefore have biases inherent to that study method. Technical variations in image acquisition and tractography reconstruction may also influence visualization of the FN across studies.
Study Characteristics and Results of Studies
Awareness of the course of the FN is crucial during surgery for VSs. Since the advent of MRI, various studies have explored the optimal imaging sequence to enhance visualization of the FN as it courses through CSF in the cisternal segment into the canalicular segment, where it might be deflected and deformed by pathology. These include T1-weighted with contrast MR sequences, as well as specialized T2-weighted sequences, which highlight fluid–tissue interfaces, such as CISS MRI, and more recently, DTI-based tractography.
Across studies, T2-based MRI sequences are more suited to delineating the course of the FN, especially when displaced by a tumor, than T1-weighted imaging, with a sensitivity of 63% to 90%.51–53 Schmalbrock et al52 could distinguish the facial or vestibulocochlear nerve branches in 63% of 27 ears with VSs, ranging from 0.06 to 3 cm3 in size, using axial T2-weighted segment-interleaved motion-compensated acquisition in steady state (SIMCAST) imaging. SIMCAST allowed for clearer differentiation of the facial-vestibulocochlear nerve complex compared to T1 contrast-enhanced techniques, while both imaging modalities were consistent in demarcating tumor size. Satoretti-Schefer et al53 corroborated the superiority of T2-weighted fast spin echo (FSE) imaging over T1-weighted contrast-enhanced sequences in discerning the FN adjacent to tumor in the CPA and IAC in 86% of 22 cases with VS. They further observed that visualization of the course of the FN diminishes with larger sized tumors, and were unable to distinguish the FN in tumors >25 mm in diameter because of either nerve thinning or obliteration of anatomic landmarks.
Other authors integrate data from both contrast-enhanced T1- and T2-weighted sequences to extrapolate the likely position of the FN based on the appearance of the intrameatal and extrameatal portions of the tumor, even when the nerve itself may not be visualized on imaging. Jung et al51 applied this strategy to 19 extra-large VSs (mean size 50 mm in diameter, range 41–70 mm) and accurately predicted the direction of FN displacement in 80% of cases, as validated by intraoperative observation. The authors attributed the few cases of false prediction to a near absence of intracanalicular tumor mass or severe destruction of the IAC, preventing an estimation of the likely direction of displacement.
Another contrast-enhanced technique used by Nakai et al54 applied gadolinium-enhanced fast-field echo (FFE) MRI to identify the FN in 46.3% of 82 patients with VSs, of which 74% (28/38) demonstrated congruence between preoperative predicted course and intraoperative observation. The authors observed that the FN was more likely to be visualized in smaller tumors, with a solid consistency.
Appreciation of the sensitivity of T2-weighted sequences to detect the FN has led to the investigation of several newer MRI modalities to further enhance the visualization of its course on preoperative imaging. In 1 study of 48 healthy subjects and 8 patients with a facial or vestibulocochlear pathology, CISS imaging successfully identified the cisternal and canalicular segments of the facial and vestibulocochlear nerves, as well as structures within the membranous labyrinth in all cases.55 Traditional T2-weighted turbo spin echo (TSE) sequences could distinguish similar segments of the FN, but had lower sensitivity in detecting individual cochlear, superior, and inferior vestibular nerves. Comparison of the spatial resolution offered by these two T2-based sequences suggested that CISS was significantly superior to TSE for visualizing canalicular segments of facial and vestibulocochlear nerves and slightly better for the cisternal segments of facial and vestibulocochlear nerves.55 Across a different cohort of 50 normal subjects and 10 patients with inner ear pathologies, the FN could be identified in the IAC in 90% of normal ears on both axial and coronal CISS sequences, most easily in the cisternal and horizontal segments, and least reliably around the posterior genu and vertical segment, where sparse CSF surrounds the nerve.56 The addition of contrast to CISS increased the ability to identify facial and vestibulocochlear nerves, as well as the discrimination between nerve and enhancing tumor in 9 patients harboring 11 VSs.57 Although contrast is not routinely added to CISS because of the hyperintense fluid signals of this T2-weighted sequence, contrast-enhanced CISS imaging was helpful in improving the distinction between nerve and immediately adjacent solid tumor, which could not be easily distinguished on precontrast CISS. In comparison, a nerve that lies against a cystic portion of a VS could be identified on noncontrast CISS.
The challenge of visualizing thinned or splayed facial-vestibulocochlear nerve fibers on standard MRI sequences when distorted by a tumor has motivated investigation into 3D fiber tractography to augment the visualization of nerves adjacent to a VS. In a proof of concept study on 3 patients with VSs, Chen et al58 reconstructed the course of the facial, trigeminal, abducens, and vestibulocochlear nerves using DTI superimposed on 3D contoured tumor volumes. The path of the facial-vestibulocochlear complex could be reconstructed in all cases, but individual contributions of the facial versus vestibular nerves within the complex could not be distinguished, nor could cisternal segment fibers in 1 case of a smaller tumor.
The sensitivity and specificity of preoperative imaging analysis of FN location has been validated by a number of studies that demonstrate excellent congruence between imaging-based prediction of FN course and intraoperative findings. In a prospective study of 11 patients with VSs, Choi et al59 correlated the FN course on preoperative diffusion tensor tractography (DTT) with intraoperative findings in all cases, and further confirmed preservation of the FN after tumor resection on postoperative tractography. The study authors achieved gross total resection in all cases. However, 91% (10/11) of patients experienced a decline in FN function early postoperatively, with 80% of those patients improving to a House–Brackmann (HB) grade II (6/10) or grade III (2/10) at 1-year follow-up.
Taoka et al60 reported a slightly lower concordance rate of 71.4% between FN course as determined on preoperative tractography when compared to intraoperative observation of the nerve trajectory in 8 patients undergoing resection of VSs. The cause of incongruence was attributed to the cystic nature of 1 schwannoma, through which the constructed tract penetrated, and the large size of another tumor, which impeded intraoperative identification of the FN. Of note, the authors were unable to reconstruct a tract that represented the FN course in a case with a smaller tumor (18 mm diameter), which was the only case where preoperative T2-weighted magnetic resonance cisternography could identify the FN. In another series of 22 patients with large VSs and normal baseline facial function, Gerganov et al61 correlated the prospective prediction of the course of the cisternal segment of the FN, in relation to the tumor, using preoperative DTT, as well as CISS imaging with intraoperative observations in 90.9% (20/22) of cases. They further correlated the morphology of the FN, defined as flat or compact, with DTI fiber patterns, but found no relation.
Ultimately, the question remains as to whether an enhanced awareness of the FN course on preoperative imaging impacts the overall clinical outcome and postoperative facial function. Kocaoglu et al62 prospectively identified the facial and cochlear nerve course in 22 patients with small VSs undergoing hearing preservation operations using both contrast-enhanced T1-weighted and CISS MRI sequences. The spatial relationship of the FN and the tumor could be determined in 82% (18/22) of cases on CISS images, but not on any of the contrast-enhanced T1-weighted sequences. The authors did not observe a correlation between the direction of the FN displacement and postoperative facial palsy or hearing loss. In comparison, Zhang et al63 identified the FN in 87.5% of 8 cases with VSs using DTT, with intraoperative concordance in all cases. They reported on the anatomic preservation of the FN with postoperative HB grade I or II function in all cases. However, operative technique and continuous intraoperative neuromonitoring also influenced the functional outcome in these cases.
Synthesis of Results
Class III evidence supports that the course of the FN may be determined on preoperative MRI, especially with T2-weighted sequences and with tractography reconstruction. However, overall functional outcome remains influenced by operative technique, philosophy, the use of neuromonitoring, and the biologic characteristics of the VS itself.
Question 3
What is the expected growth rate of vestibular schwannomas on MRI, and how often should they be imaged if a “watch and wait” philosophy is pursued?
Target population
Adults with suspected vestibular schwannomas by imaging
Recommendation
Level 3: MRIs should be obtained annually for 5 years, with interval lengthening thereafter with tumor stability.
Study Selection
For this section, 25 full-text articles were reviewed after an initial analysis of 88 abstracts. Of these, 15 were included as evidence (Table 5). Articles were excluded because of the predominant use of CT, small numbers of patients, and a lack of focus on growth or the assessment of growth after treatment.
Risk of Bias and Limitations
The majority of studies are retrospective. The accurate comparison of studies is confounded by the variable definition of growth, the inclusion of both static and growing tumors, patient selection within cohorts, and the method of tumor measurement.
Study Characteristics and Results of Studies
A significant amount of literature has documented the natural history of untreated sporadic VSs, with average rates of growth cited as 1.2 to 1.9 mm/year in systematic literature reviews.64,65
Stangerup et al66 published the largest study on the growth rate of sporadic VSs; these are especially unique because all the patients with VSs were evaluated in a single center in Denmark in a prospective fashion. Of 552 patients who had ≥2 MRI scans since 1989, the mean observation period was 3.6 years. Their group, in general, conservatively manages intrameatal tumors and extrameatal tumors <2 cm. The authors defined the growth of intrameatal tumors as growth to extrameatal extension, and of extrameatal tumors by an increase in size of ≥2 mm.
In intrameatal tumors, 83% of the tumors remained in the meatus during the observation period. Thirty-nine tumors (17%) fulfilled the criteria for growth by growing to the extrameatal extension. During the first year of observation, growth was observed in 25 of 39 (64%) patients, with an average growth rate in these tumors of 10.3 mm/year. Fewer tumors were detected to be enlarging in successive years, and no tumors initiated tumor growth after the fourth year. Growth rates were highest if growth was detected in the first year.
In extrameatal tumors, 70.2% of tumors were unchanged in size, 28.9% increased in size, and 0.9% decreased in size. A similar trend was observed in these tumors compared to intrameatal schwannomas. In 62% of growing tumors, the growth was detected during the first year, and these tumors had faster growth rates (4.9 mm/year). In the second year, growth was determined in 26% with a mean growth rate of 2.79 mm/year; in the third year, growth was determined in 10% with a mean growth rate of 1.15 mm/year; and in the fourth year, growth was noted in 2% with a mean rate of 0.75 mm/year. As in intrameatal tumors, no growth was observed after the fourth year of observation.
Overall, 29% of extrameatal tumors fulfilled the criteria of growth compared with 17% of intrameatal tumors. The authors generally recommend yearly MRI for 5 years, followed by MRI every other year for 4 years, followed by MRI after 5 years, after which the observation is terminated provided that no growth has occurred.
Flint et al67 followed 100 patients for a median of 25.5 months with tumor size <24 mm. Of these, 62% showed no growth. Of growing tumors, 80% grew within the first year; of those that grew initially, 66% continued to grow. Interestingly, 20% of patients whose tumors grew did not grow initially had a latency period of between 8 to 60 months before growth, suggesting a need for continued vigilance. Growing tumors enlarged on average 2.68 mm/year. Initial size did not predict future growth. This was similar to findings from Hoistad et al,68 who reviewed 102 patients followed conservatively for a mean of 28.5 months with ≥2 MRI scans; 44% of patients showed growth (average 2.17 mm), and the presenting tumor size was not predictive of future growth, similar to findings from Bozorg-Grayeli et al.69
Other groups with large cohorts of patients have also reported their observations, with some making recommendations on scanning intervals. Moffat et al,70 in a cohort of 381 patients with small- to medium-sized sporadic VSs managed conservatively with ≥2 MRI scans, defined growth as the mediolateral diameter changing by ≥2 mm on successive scans. Overall, 59.3% of tumors did not change in size, 32.5% of tumors increased in size, and 8% regressed. While the average growth was 0.7 mm/year overall, growing tumors on average enlarged 2.3 mm/year. 23.5% of intrameatal tumors extended to the CPA on follow-up.70 In general, most tumors displaying a growth phenotype showed growth within 3 years of presentation; however, 7% of tumors showed growth after 5 years. Growth rates slowed over time. Patients tended to follow differing growth patterns. For patients who experienced growth then quiescence, the mean duration of initial growth was 15.6 months. For those tumors that did not grow initially but then started to grow, the mean time until growth was demonstrated was 27.4 months. Approximately 13% of these tumors started growing after 5 years of no growth, one third of which started growing after 9 years. The authors recommend an MRI 6 months after initial diagnosis followed by annual scans, at which point scans can be done every 2 years for 6 years, and then every 3 years if growth is not documented. Similarly, in Martin et al’s study,71 which followed 276 patients with ≥1 follow-up MRI, 78% were quiescent, and tumor growth occurred within 3 years. Rapidly growing tumors did show evidence of growth at 6 months.
Suryanarayanan et al72 reported similar findings in a cohort of 240 patients for whom ≥2 MRI scans were available with a mean follow-up of 3.6 years. Overall, 68% of tumors did not grow (their study used a more stringent growth criterion of change in diameter of 1 mm). Thirty percent grew, and 2% regressed. Intrameatal tumors were less likely to grow, and tumors with a cisternal size of >15 mm were more likely to continue growing. No specific scan interval recommendations were provided. Similar rates of tumor quiescence have been reported by others. Fucci et al73 followed a cohort of 119 patients with a mean size of 1.0 cm for an average of 2.5 years with ≥2 MRI scans, noting that 66% of patients did not meet growth criteria (>2 mm). The average growth rate overall was 1.2 mm, but growing tumors grew at a rate of 3.8 mm/year. Tumor size portended growth in this cohort; 71% of tumors >20 mm grew. Only size at initial presentation predicted future growth. The authors recommend an interval scan 6 months after the initial scan to identify growing tumors. In their follow-up study of this cohort of patients,74 37.7% of tumors had grown at 5 years, with a mean growth rate of 3.1 mm/year. Overall, 51.7% of patients whose tumors had grown demonstrated growth at 1 year after initial MRI; 22% of growing tumors showed growth at 6 months. After 2 years of no growth, only 12% of tumors grew thereafter; after 5 years of no growth, 4% of tumors showed growth. Monitoring by MRI is typically scheduled at 6 months after initial visit, at 1 and 2 years after that, at 5 years, and then only if symptoms change. Tumor growth is very unlikely (~4%) if no growth has been observed by the 5-year follow-up.
A similar 5-year no-growth rate (NGR) was reported by Solares et al,75 who reviewed 110 patients who had been managed conservatively with ≥2 MRIs over a mean follow-up period of 31.4 months. Overall, the 5-year NGR was 70.6%; intracanalicular tumors had a no-growth rate of 89.8%. Smaller tumors (≤10 mm of extrameatal component) had a NGR of 73.9%, and grade II or larger, 45.2%. Therefore, larger tumors were more likely to grow in their series. The authors offer conservative treatment to patients with extracanalicular tumors <15 mm.
Bakkouri et al76 reviewed a cohort of 325 patients in whom ≥2 serial MRIs had been performed. The first MRI study was performed 1 year after diagnosis, with successive scans at 1- or 2-year intervals.76 Twelve percent of tumors grew >3 mm in 1 year and were treated, so 286 patients were available for further study. The overall growth rate was 1.15 mm/year; 57.8% showed no growth overall and 87.8% had either no growth or growth <3 mm. Intra- and extrameatal growth rates were similar. Of 174 intrameatal tumors, 39% remained intrameatal at 3 years. Shorter duration of symptoms was associated with a failure of conservative management. Tumor growth was hard to predict. Even at the 7-year follow-up, 3 of 21 (14%) patients showed tumor growth; at 9 years, 1 of 8 patients (12.5%) showed growth. The duration of symptoms was also observed to predict growth in other reports. Tschudi et al,77 in their cohort followed for a mean of 35 months, noted a 68.9% NGR, but added that growth in the first year was significantly predictive of future growth. Moreover, in their series, patients with progressive hearing loss were associated with slower growing tumors.
Ferri et al78 followed 123 patients conservatively per their treatment algorithm, scanning each patient 6 months after their initial scan. Overall, 64.5% of tumors showed no increase in size growth (defined by changed >2 mm); 6% showed growth reduction. Of growing tumors, 45.4% grew within the first year and 22.7% grew at least 3 years after the initial scan. No growth occurred after 6 years. Growing tumors grew 1.2 mm/year. Intracanalicular tumors were less likely to grow. Symptoms >10 years portended no growth, but tinnitus as an initial symptom was predictive of growth. Other authors have correlated symptoms at presentation with likelihood of growth. Artz et al79 reviewed a prospectively collected group of 234 patients with sporadic unilateral VSs, with those managed conservatively having ≥2 MRI scans followed over a mean of 28 months with a view towards developing a risk profile for predictors of growth. Growth was defined as change in axial diameter of ≥1 mm. The authors suggest that initial symptoms can assist in predicting risk of growth. Risk factors for growth included extrameatal location, and among symptoms, tinnitus, unsteadiness/vertigo, no sudden sensorineural hearing loss (SSHL), and short duration of hearing loss (1–24 months). In their model, “high risk” tumors were either extrameatal with short duration of hearing loss and either unsteadiness/vertigo or no SSHL, or were intrameatal with short duration of hearing loss, unsteadiness/vertigo, and no SSHL. In this group, the risk of growth was 36.9% in the first year and 64.6% in 2 years. Low risk tumors were extrameatal with no other risk factors or intrameatal with at most 1 other risk factor. In this group of tumors, the risk of growth was 2.5% in the first year and 12.7% within the first 2 years.
Varughese et al80 suggested an alternative means of predicting growth by assessing volume doubling time (VDT) rather than linear measurements. Their cohort consisted of 178 patients followed prospectively with a mean follow-up of 35 months and an average of 3 scans per patient to establish growth rates.80 A VDT of 5.22 years was highly predictive of discrimination between growing and nongrowing tumors.
Synthesis of Results
Class III evidence support the conclusion that about two-thirds of patients with VSs may not exhibit measurable growth, while one-third demonstrate growth, defined variably as either any increase in size or a change in diameter > mm. Intrameatal tumors are less likely to grow. While large literature surveys suggest average growth rates of 1.2 to 1.9 mm/year, separate analysis of actively growing tumors reveal faster rates. Early growth may predict future growth; however, late growth after 5 years of quiescence may occur. An MRI 6 months after tumor discovery may identify tumors likely to continue growing; otherwise, scans may be obtained annually for 5 years, and scan intervals should be lengthened if no growth is detected.
Question 4
Do cystic vestibular schwannomas behave differently than their solid counterparts?
Target population
Adults with vestibular schwannomas with cystic components
Recommendation
Level 3: Adults with cystic vestibular schwannomas should be counseled that their tumors may more often be associated with rapid growth, lower rates of complete resection, and facial nerve outcomes that may be inferior in the immediate postoperative period but similar to non-cystic schwannomas over time.
Study Selection
Thirty-four articles were initially identified for analysis of cystic VSs in the MRI era. Of these, 19 were chosen for final discussion based on relevance (Table 6).
Risk of Bias and Limitations
Studies were limited by their retrospective nature, a variable definition of what constitutes a “cystic schwannoma,” and variable follow-up.
Study Characteristics and Results of Studies
VSs with cystic radiographic features are perceived to represent a more formidable variant than their solid counterparts. Cystic VSs are often thought to grow faster, be more adherent to the FN, and are associated with worse outcome. The authors sought to investigate this question in the literature.
While the specific definition of cystic tumor differs among reports, cystic schwannomas account for 4% to 24% of tumors in the literature.26–32 Some groups have attempted to classify the cystic appearance and delineate cystic configurations that may confer greater operative difficulty and worse outcome. Benech et al33 noted anteriorly placed cysts to be more challenging. Metwali et al34 classified cysts as either multiple large thin-walled cysts, multiple small thick-walled cysts, single large thin-walled cyst, large central thick-walled cyst, or a mixed pattern of small and large cysts. They noted medially located thin-walled cystic tumors to be the most difficult to handle. Piccirillo et al35 classified cysts as to whether they were central and thick-walled (type A) or peripheral and thin-walled (type B), concluding that type B cysts presented a greater clinical challenge. When examining the volume burden contributed by the cystic portion, Mehrotra et al36 noted the increasing difficulty of FN dissection if the tumor was ≥90% cystic.
Cystic schwannomas tend to be large. Metwali et al34 reported that their 37 cases were all Hanover T4 tumors. Larger medial VSs were nearly all cystic in another report,37 and additional studies have reported average diameters of 2.5 to 6.2 cm.26,27,30,31,35 Their growth rates and symptomatic manifestations may also accelerate more rapidly than solid tumors as reported variably by Charabi et al,26 Benech et al,33 Sinha et al,38 and Mehrotra et al.36 The duration of symptoms at the time of discovery was shorter in some reports,33,38 while Mehrotra et al36 and Benech et al33 reported a 27% and 19% rate, respectively, of significant clinical worsening in patients with cystic tumors.
The extent of resection has been reported as similar to lower for cystic schwannomas compared to similarly sized solid schwannomas. Piccirillo et al35 observed a similar rate of complete resection between cystic and solid tumors (82% vs 84%). Others have corroborated comparable rates of resection,27,31,33 while some reported lower rates of complete resection when compared to solid tumors (76% vs 90.2%).36,38
Cystic schwannomas are associated with equivalent or worse FN outcomes than solid tumors, with outcomes dependent on the duration of follow-up after surgery. In a landmark series of 1000 patients, Samii et al39 noted a lower rate of FN preservation in cystic tumors. Metwali et al34 reported HB grade I to III FN function in 62.1% of cystic schwannomas compared to 82.5% of solid tumors of similar size early postoperatively, with equilibration to a nearly identical incidence at 1 year after surgery (91.8% vs 93.8%). Zaouche et al32 also noted a higher rate of FN palsy in cystic schwannomas in the early postoperative period, and Mehrotra et al36 report a lower rate of grade I to II function in a similar timeframe. Fundova et al,27 however, reported inferior FN outcomes at 1 year in patients with cystic tumors when compared to patients with solid tumors, noting a statistically significant increase in grade VI function (P = .04).40 Sinha et al38 also reported superior FN outcomes in patients with solid tumors at 6 months, with grade I to III in 67.9% of patients with cystic tumors versus compared to 82.7% with solid tumors. Piccirillo et al,35 in comparing surgical results of 57 cystic schwannomas to solid schwannomas at 1 year, reported an 81% rate of grade I to III function (with a trend towards grade III function), which was similar to patients who had solid tumors.
A near equivalence in facial function at longer follow-up in patients with cystic tumors has also been reported in other series. Jones et al41 note that function at 2 years is not statistically different in a matched cohort of 70 patients, and Benech et al33 note similar rates of grade I to III function at 1 year. In the former study, however, more patients have grade VI function who had cystic tumors, and fewer patients with cystic tumors had grade I function. In summary, FN outcomes may be worse in the early postoperative period. With longer-term follow-up, these results sometimes equilibrate.
A higher incidence of postoperative hemorrhage and hydrocephalus is associated with cystic schwannomas in some studies. Metwali et al34 reported an 8.1% rate of postoperative hematoma compared to 1.7% in solid tumors, and a higher rate of hydrocephalus, with other groups reporting higher rates of complication rates as well.27,42
Lastly, an emerging literature highlights the behavior of cystic tumors after irradiation. While some have reported the need for surgery because of symptomatic enlargement of the cystic component in a small number of cases after radiosurgery,43 other groups have reported good control rates, albeit with fractionation. In a group of 65 tumors, 20 of which were cystic with a mean size of 2.1 cm, Shirato et al44 reported a 3-year tumor reduction rate of 31% for solid tumors and 93% for cystic tumors, despite an early increase in the size of cystic tumors within the first 2 years of treatment.
Synthesis of Results
Cystic VSs are variably defined and represent between 4% and 24% of tumors in most series. Class III evidence supports the conclusions that they may demonstrate rapid growth, symptomatic deterioration, and be associated with lower rates of complete resection, worse short-term FN outcomes, and unpredictable response to radiation. Long-term FN outcomes may be equivalent to solid VSs.
Question 5
Should the extent of lateral internal auditory canal (IAC) involvement be considered by treating physicians?
Target population
Adult patients with a vestibular schwannomas
Recommendation
Level 3: The degree of lateral IAC involvement by tumor adversely affects facial nerve and hearing outcomes and should be emphasized when interpreting imaging for preoperative planning.
Study Selection
Nineteen full-text articles were reviewed, and 13 were excluded (7 did not address lateral extent of IAC involvement, 4 addressed imaging of lateral IAC but did not discuss decision-making based on imaging, and the data pertinent to the lateral extent of VSs were not extractable in 2 studies; Table 7).
Risk of Bias and Limitations
All studies that were included in this analysis were retrospective and therefore had biases inherent to that study method. Bias has the potential to be amplified across these analyses, as individual surgeons’ technical skills and experience are of particular importance in facial and cochlear nerve preservation after VS surgery.
Study Characteristics and Results of Studies
Gerganov et al45 retrospectively reviewed 99 consecutive VSs and evaluated the impact of preoperative tumor volume, width, and length on postoperative FN function. Increasing tumor volume, extrameatal tumor volume, and increasing Hannover stage all correlated with worse postoperative HB scores (P < .05). Intrameatal tumor length/width, as well as tumor-fundus distance showed no impact on postoperative HB scores. Rompaey et al46 retrospectively evaluated postoperative HB scores in 123 consecutive patients with and without fundal obliteration on preoperative MRI. In the short term, 1-month postoperative HB scores ≥3 occurred in 29.7% of patients with complete fundal obliteration compared to 13.0% with no fundal obliteration. No statistically significant difference between the groups was found at 1-year follow-up. Kobayashi et al47 retrospectively evaluated fundus distance from small ANs and normal preoperative FN function in 45 patients; distance from fundus and tumor diameter had no effect on FN function at 2 weeks or at 3 months postoperatively.
Lateral IAC involvement by tumor is hypothesized to negatively influence cochlear nerve function. Gerganov et al48 retrospectively reviewed 99 consecutive VSs (the same study population as previously discussed in this section) and showed that the degree of intrameatal tumor growth was significantly correlated with the level of preoperative hearing assessed by the Hannover scale. A shorter distance between the lateral tumor margin and the fundus was significantly correlated with worse preoperative Hanover score. Matthies et al49 retrospectively evaluated CT parameters in 202 VSs. The length of posterior auditory canal and the maximum porus width both correlated with the degree of postoperative hearing deterioration. The extent of widening of the IAC was of predictive importance for postoperative hearing preservation (P < .01). Mohr et al50 retrospectively evaluated the impact that the extent of filling of the IAC and the size of the VS had on serviceable hearing in 128 consecutive cases. Incomplete filling of the IAC and smaller size (<15 mm) proved statistically significant for serviceable hearing preservation (P = .026 and P < .001, respectively).
Synthesis of Results
Class III evidence supports the conclusion that lateral involvement correlated with decreased FN function, at least in the short-term.45,47 The extent of lateral IAC involvement appears to correlate with worse preoperative and postoperative hearing outcomes.
Question 6
How should patients with neurofibromatosis type 2 (NF2) and vestibular schwannoma be imaged and over what follow-up period?
Target population
Adult patients with neurofibromatosis type 2 and vestibular schwannomas
Recommendation
Level 3: In general, vestibular schwannomas associated with NF2 should be imaged (similar to sporadic schwannomas) with the following caveats:
- More frequent imaging may be adopted in NF2 patients because of a more variable growth rate for vestibular schwannomas, and annual imaging may ensue once the growth rate is established.
- In NF2 patients with bilateral vestibular schwannomas, growth rate of a vestibular schwannoma may increase after resection of the contralateral tumor, and therefore, more frequent imaging may be indicated, based on the non-operated tumor’s historical rate of growth.
- Careful consideration should be given to whether contrast is necessary in follow-up studies or if high-resolution T2 (including CISS or FIESTA-type sequences) MRI may adequately characterize changes in lesion size instead.
Study Selection
Twenty-one full-text articles were reviewed, and 9 were excluded (5 because of an emphasis on radiotherapy technique and success rate, 3 because of an emphasis on hearing preservation, and 1 for addressing a pediatric population; Table 8).
Risk of Bias and Limitations
All but 3 included studies were retrospective. In addition, some older studies based growth determination on thin-section imaging that did not use isotropic voxels, potentially compromising measurement accuracy.
Study Characteristics and Results of Studies
Numerous studies have characterized the rate of growth of VSs in NF2 patients. The technique used to determine growth has received some attention. When volumetric techniques are available, these are shown to have greater accuracy for detecting smaller degrees of change than simple diameter-based volume calculations.94,95 Comparisons between local radiologist and neuroradiologist measurements showed good agreement (kappa = 0.77), but neuroradiology expertise offered superior measurements for smaller tumors (<5 mm) and in postoperative imaging.96
Slattery et al97 found that VSs in NF2 increased 1.3 mm/year on average in short-term follow-up, but a minority (8%) may exhibit growth of ≥5 mm over 4 years. The pattern of growth for VSs is most frequently saltatory (47%), characterized by periods of quiescence (lasting 2.8 ± 2.2 years, range 0.4–6.9 years) punctuated with bursts of growth; exponential growth (40%) and linear growth (13%) were less common. In fact, saltatory growth is the most common pattern among all intracranial NF2-associated tumors, including meningiomas and schwannomas of both vestibular and nonvestibular origin (59% saltatory, 30% linear, 11% exponential).98
The growth rate of NF2-associated VSs differs based on the age at presentation. The natural growth rate in the pediatric population is slow but may accelerate once patients enter adulthood.99,100 A majority of elderly NF2 patients who present with VSs (8/11, 72%) exhibited no significant growth after a mean follow-up period of 8 years.101 This suggests that older patients might be reimaged less frequently than younger patients.
When one of bilateral schwannomas is resected, the rate of growth of the remaining VS may increase.92,102 One study observed a near doubling of the nonoperated tumor growth rate following resection of the contralateral tumor (4.4 ± 3.3 mm/year compared with 2.5 ± 2.2 mm/year preoperatively).92 This may lead clinicians to pursue imaging more frequently than if neither of the bilateral schwannomas has been resected.
Following radiosurgery, the vast majority of schwannomas in NF2 (≥94%) either remain stable (62%) or regress (36%) over the subsequent 3 to 5 years.103,104 Mallory et al105 found that 84% of NF2-associated VSs exhibit growth arrest at a median follow-up of 7.6 years, but also noted how marginal dose also plays a role: the median marginal dose for tumors decreasing in size was 15.5 Gy while the median marginal dose for enlarging tumors was 13 Gy.
Historically, follow-up imaging after stereotactic radiosurgery (SRS) may have been performed at 6-month intervals for ≤2 years, annually for the next 3, and biannually thereafter.97 The rationale would identify relatively faster growing lesions early, to initiate therapy, while still imaging at lower frequency later to capture slower growing lesions. Ultimately, when determining response, measurements should take into account the posttreatment enlargement that is characteristically seen within the first 1 to 2 years.
Overall, the current literature does not offer significantly different imaging recommendations between NF2 and non-NF2 patients. With any protocol, imaging should be pursued when new symptoms arise or if resection is contemplated.98 Depending upon patient age, monitoring may be eliminated or reduced in older populations if stability is established and the likelihood of lifetime growth declines.
Synthesis of Results
Class III evidence supports the conclusion that NF2-associated VSs demonstrate unique growth patterns and growth rates. Although lesions that grow linearly may enlarge only 1 to 2 mm/year, the saltatory growth pattern is often observed with periods of quiescence punctuated by periods of rapid growth. Even if some clinicians recommend tapering surveillance after periods of instability, these characteristics suggest that there should be a low threshold for imaging should a patient become symptomatic. When measuring lesions currently, an effort should be made to determine the size from volumetric analysis.95 Specific follow-up paradigms may be similar to non-NF2 tumors. However, the reported growth rate of a nonoperated tumor in cases of bilateral tumors where one has been resected may lead to more frequent imaging.92 After SRS, most (94%) schwannomas remain stable or regress over the next 3 to 5 years.
Question 7
How long should vestibular schwannomas be imaged after surgery, including after gross total, near total, and subtotal resection?
Target population
Adult patients with vestibular schwannomas followed after surgery
Recommendation
Level 3: For patients receiving gross total resection, a postoperative MRI may be considered to document the surgical impression and may occur as late as 1 year after surgery. For patients not receiving gross total resection, more frequent surveillance scans are suggested; annual MRI scans may be reasonable for 5 years. Imaging follow-up should be adjusted accordingly for continued surveillance if any change in nodular enhancement is demonstrated.
Study Selection
Twenty-eight full-text articles were reviewed, and 15 papers were included for discussion (Table 9). Excluded articles did not offer sufficient data or recommendations regarding follow-up imaging.
Risk of Bias and Limitations
The majority of these studies were retrospective and were therefore subject to the inherent bias associated with any retrospective analysis. They also largely emanated from single institutions, often from a single surgeon. The studies do not consistently include, exclude, or discuss the possible confounding factor of patients with NF2. Follow-up was variable. Finally, the conclusions given by each study reviewed were subject to the limitations present with expert opinion.
Study Characteristics and Results of Studies
Variability exists regarding the frequency of surveillance in patients undergoing treatment for VSs after complete or incomplete resection. Several groups report MRI findings in cohorts of patients with varying degrees of extent of resection. Bennett et al25 described 299 patients who underwent various approaches for surgical resection of VSs and had 1- and 5-year follow-up MRI scans. They report complete resection in all but 2 patients with NF2 that underwent near-total resection. Linear enhancement was noted in 10 patients without any noted enlargement; 3 patients had nodular enhancement with 2 undergoing enlargement and recurrence. Tysome et al81 evaluated 314 consecutively treated patients followed in a prospectively maintained database who underwent complete translabyrinthine excisions of VSs and had follow-up imaging at 2 and 5 years after surgery. All patients in whom MRI was reported to show no recurrence at 2 years (97%) also had no signs of recurrence on MRI at 5 years. All 8 patients with MRI suspicious of recurrence (linear enhancement of IAC) at 2 years had no progression on MRI at 5 to 15 years. One patient had nodular enhancement within the IAC at 2 years, with the authors concluding that patients where nodular enhancement is seen should be considered to have residual or recurrent disease. If MRI shows linear enhancement, patients should have another MRI at 5 years. If this shows no progression, further imaging may not be required. Schmerber et al82 reported on 91 patients who received gross total resection (GTR), suggesting 1 single MRI at 5 years for patients who received GTR. If any enhancement is seen, they recommend a 2-year follow-up thereafter. Carlson et al21 report 203 non-NF2 patients who underwent surgical resection and at least two follow-up MRIs (mean 2.6); 144 received GTR and 59 subtotal resection (STR; near-total resection [NTR] was not distinguished). Nodular enhancement—especially ≥15 mm in diameter—on the initial postoperative MRI was associated with a 16-fold increased risk for future recurrence when compared to linear contrast enhancement.
Arlt et al83 conducted a study of 50 patients who underwent resection that received follow-up MRIs at 3 months and then yearly. Of the 28 patients receiving STR, 9 showed progression of tumor remnant at a median time of 44 months. Of the 22 patients receiving GTR, 2 patients had recurrence at a median of 41.5 months. Fukuda et al84 conducted a review of 74 patients who underwent resection with follow-up MRIs at 3 to 6 months, 12 months, and then yearly. Of the 41 patients receiving GTR, 25 patients receiving STR, and 8 patients receiving partial resection (<90% tumor removal), 1, 13, and 5 patients recurred, respectively. Godefroy et al85 reported on 50 patients who underwent translabyrinthine resection of VSs with 13 GTR, 29 NTR, and 8 STR. Their reported follow-up protocol was first postoperative MRI at 11 ± 7 months, followed by a second surveillance MRI at 29 ± 9 months and a final MRI at 49 ± 17 months. They reported no tumor recurrences during this follow-up period. Tang et al86 discuss 88 patients with mean MRI follow-up after surgery of 3.9 years. There were 46 complete resections and 42 incomplete resections. While they do not comment explicitly on recurrence they discussed evolution of various enhancement patterns over time and make the following recommendations: obtain a baseline MRI at 6 months postoperatively; for patients with linear or no enhancement on the baseline scan, no further imaging until 5 years postoperatively unless the patient develops new symptoms; for patients with nodular enhancement on the baseline scan, they recommend obtaining annual MRIs starting at 2 years postoperatively and offering further treatment when MRI shows an increase in enhancement of 97 mm2 in maximal axial area or >133 mm3 in volume. Not all nodular enhancement portends recurrence. Carlson et al22 reported on 16 patients treated with surgery for VSs at their tertiary referral center who demonstrated unusual enhancement in the IAC lateral to preoperative tumor bed after GTR. Following their general institutional protocol of postoperative imaging (initial MRI at 3 months, and then 2, 7, and 17 years if all negative, vs annual MRI for at least 2 or 3 years [until stable] if the 3-month postoperative MRI reveals enhancement), these 16 patients were followed for an average of 39.8 months without any evidence of recurrence.
Several authors also report on MRI findings in patients with intentional or unintentional incomplete resections. Lemee et al87 conducted a review of 33 (30 non-NF) patients with a postsurgical VS tumor remnant [87]. Two non-NF patients had tumor remnant growth which occurred at 38 and 58 months after surgery. The authors make the following recommendations: if a postoperative VS remnant seen on first MRI 3 months after surgery in a non-NF2 patient, and remnant >1.5 cm3 or postoperative FN function >4 on the HB scale, then consider SRS. If these additional factors are not seen, clinical and radiologic follow-up should occur yearly for 6 years, then once every 2 years. Bloch et al88 report on 79 patients with either STR or NTR via various surgical approaches. The rate of recurrence was 3% for NTR and 32% for STR occurring at a mean time interval of 3 years postoperatively (recurrence being defined as either remnant growth on serial scans or when patients underwent additional tumor treatment following one scan). The authors describe their imaging protocol as surveillance MRI at 1 and 3 years postoperatively in GTR patients, and annual imaging in patients not receiving GTR with a gradual lengthening of interval between scans if no evidence of recurrence. Kameyama et al89 conducted a cohort study of 11 patients who were known to have received subtotal resection of VSs with known intracanalicular remnant and performed a single long-term follow-up MRI to measure the fate of these remnants. Of the 11 patients imaged, 2 had no evidence of tumor remnant, 6 had a small intracanalicular tumor, and 3 had intracanalicular tumor with slight protrusion into the intracranial compartment. None of these patients required reoperation. A similar cohort study of 14 patients known to have received subtotal resection of VSs with known “tumor capsule” remnant who underwent a single follow-up MRI90 showed enhancing tumor remnant in 7 patients. None of these patients had clinical signs suggesting tumor regrowth or required reoperation. For patients with suspected tumor remnant at time of surgery, the authors recommend a postoperative MRI at 3 months followed by another at 2 years and variable times thereafter, determined by presence or absence of enhancement as well as patient age. Kemink et al91 conducted a review of 20 patients who underwent surgery with intentional STR (n = 8) or NTR (n = 12). Follow-up protocol used included postoperative contrasted CT or MRI at 2 months, followed by a yearly scan thereafter. Mean length of follow-up was 5 years. Radiographically detectable tumor regrowth occurred in only 1 patient (in the STR group).
Peyre et al92 report on a very specific scenario with a case series of 11 NF patients with bilateral VSs who undergo an initial unilateral resection, describing 5 patients who required operation on the contralateral tumor at a median of 1.8 months because of radiographically confirmed accelerated growth compared before the initial operation. They conclude by recommending 6-month follow-up after resection one of a bilateral pair of VSs in NF patients.
Interestingly, a clinical practice survey by Lee et al93 assessing MRI acquisition patterns among 88 neurotologists (NOs) and 47 neurosurgeons (NSs) revealed that the average number of postoperative MRIs obtained by NOs was 3.6 and 5.6 for NSs, with only 2.3% of NOs obtaining an MRI on postoperative day 1 versus 23.4% of NSs. Only 21.6% of NOs obtained an MRI within the first year postoperatively vs 61.7% of NSs. 35% and 32% of NOs and NSs ended their follow-up imaging at 5 years respectively, and 16% of NOs and 11 % of NSs stopped at 10 years.
Synthesis of Results
There is striking variation among the frequency of recurrence in the various reported groups which each represented a single surgeon’s or a single institution’s experience. Class III evidence support the conclusions that a first MRI for a GTR can reasonably take place within 6 months after surgery; the first MRI for a non-GTR should certainly occur before 6 months postsurgery A final MRI for GTR patients should take place at 5 years from surgery, with optional MRI follow-up if both the 2- and 5-year scans are without nodular enhancement. If a non-GTR patient shows persistent enhancement on scans without progression, MRI should be obtained yearly with decreasing frequency towards a final MRI at approximately 10 years. Any progressive or new nodular enhancement should prompt greater surveillance frequency of a repeat MRI every 6 to 12 months until further treatment is undertaken or the enhancement stabilizes over multiple images.
Discussion
VSs are usually imaged with MRI, with contrast-enhanced scans generally considered the criterion standard for the initial evaluation and postoperative assessment of recurrence or residual tumor. The use of high-resolution T2 sequences to follow VSs, however, is supported by class II evidence. Specific imaging features with clinical application—though supported by class III evidence—include, in addition to size and the presence of hydrocephalus, the extent of lateral extension in the IAC and the presence of cystic intratumoral contents.
Growth rates of conservatively managed tumors are well established, with most tumors quiescent, particularly those confined to the IAC. Importantly, early growth appears to predict future growth, but a small percentage of patients may show initial growth after 5 years, justifying long-term surveillance for patients with VSs. Patients with NF2 require particular scrutiny. Long-term radiologic follow-up is particularly important in the event of incomplete resection or should nodular enhancement be noted on MRI.
Conclusions and Key Issues for Future Investigations
Imaging is a crucial tool in the evaluation and management of patients with VSs, with MRI supplanting CT nearly entirely. Its higher resolution and iterative use over time in conservatively managed and treated patients in large centers has allowed spatial constructions of the natural history of these tumors, arming patients and physicians alike with important information in considering treatment and monitoring. Bias as to which patients are treated conservatively and interobserver variation in the definition of growth rate may underlie differential reporting. This could be ameliorated with automated size calculations which include standard measurements of cisternal and intracanalicular components in 2D and even volumetric fashion, which larger prospective comparative studies could validate.
Higher-resolution T2 sequences and refinements in DTI may render FN identification even more reliable, providing valuable information to the surgeon—and in turn the patient—preoperatively. The contribution of reliable imaging identification of the FN course in VSs to surgical outcomes remains unclear. These sequences may become more relevant in light of nascent concerns over gadolinium retention in the brain; clarification of the clinical relevance of this deposition could lead to a more rational use of contrast administration in the MRI follow-up of patients with VSs.
Conflict of Interest (COI)
The Vestibular Schwannoma Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript (here).
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Committee for its review, comments, and suggestions throughout peer review, as well as Trish Rehring, MPH, CHES, and Mary Bodach, MLIS, for her assistance with the literature searches. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Sepideh Amin-Hanjani, MD, D. Ryan Ormond, MD, Andrew P. Carlson, MD, Kimon Bekelis, MD, Stacey Quintero Wolfe, MD, Chad W. Washington, MD, Cheerag Dipakkumar Upadhyaya, MD, and Mateo Ziu, MD.
Figures
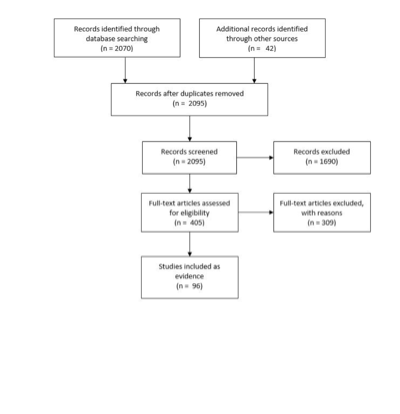
Figure 1. Article flow chart.
Table 1. Search strategy
|
PubMed (NCBI) Search
|
|
Step 1. Neuroma, Acoustic [MeSH]
|
|
Step 2. (vestibular [Title/Abstract] OR vestibulocochlear [Title/Abstract] OR acoustic [Title/Abstract]) AND (neuroma* [Title/Abstract] OR neurilemmoma* [Title/Abstract] OR neurilemoma* [Title/Abstract] OR neurinoma* [Title/Abstract] OR tumor* [Title/Abstract] OR tumour* [Title/Abstract] OR schwannoma* [Title/Abstract])
|
|
Step 3. Step 1 OR Step 2
|
|
Step 4. Diagnostic imaging [MeSH] OR Radiography [SH] OR Radionuclide imaging [SH] OR Ultrasonography [SH]
|
|
Step 5. Magnetic resonance imaging [TIAB] OR MRI [TIAB] OR Computed tomography [TIAB] OR CT [TIAB] OR Positron emission tomography [TIAB] OR PET [TIAB] OR FDG [TIAB] OR MET [TIAB] OR FET [TIAB] OR Diffusion tensor imaging [TIAB] OR DTI [TIAB] OR CISS [TIAB] OR FIESTA [TIAB] OR Spectroscop* [TIAB] OR SPECT [TIAB] OR imag* [TIAB] OR radiograph* [TIAB]
|
|
Step 6. Step 4 OR Step 5
|
|
Step 7. Step 3 AND Step 6
|
|
Step 8. Step 7 AND English [Lang]
|
|
Step 9. (animal [MeSH] NOT human [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR case reports [PT]
|
|
Step 10. Step 8 NOT Step 9
|
|
Step 11. Step 10 AND (“1946/01/01” [PDAT] : “2015/01/01” [PDAT])
|
Cochrane CENTRAL Search
|
|
Step 1. MeSH descriptor: [Neuroma, Acoustic] explode all trees
|
|
Step 2. ((vestibular or vestibulocochlear or acoustic) and (neuroma* or neurilemmoma* or neurilemoma* or neurinoma* or tumor* or schwannoma*)):ti,ab,kw
|
|
Step 3. Step 1 OR Step 2
|
|
Step 4. MeSH descriptor: [Diagnostic Imaging] explode all trees
|
|
Step 5. Any MeSH descriptor with qualifier(s): [Radiography - RA]
|
|
Step 6. Any MeSH descriptor with qualifier(s): [Radionuclide imaging - RI]
|
|
Step 7. Any MeSH descriptor with qualifier(s): [Ultrasonography - US]
|
|
Step 8. "Magnetic resonance imaging" or MRI or "Computed tomography" or CT or "Positron emission tomography" or PET or FDG or MET or FET or "Diffusion tensor imaging" or DTI or CISS or FIESTA or Spectroscop* or SPECT or imag* or radiograph*:ti,ab,kw
|
|
Step 9. Step 4 or Step 5 or Step 6 or Step 7 or Step 8
|
|
Step 10. Step 3 and Step 9
|
|
Publication dates 1946-2014
|
|
Total articles reviewed: 2,070
|
Table 2. Magnetic resonance imaging assessment of vestibular schwannoma: Initial diagnosis
|
Author, Year
|
Description of Study
|
Data Class
|
Conclusions
|
|
Singh et al, 2015
|
Prospective study of 19 patients who underwent MRI (standard protocol of T1WI, T2WI, DWI, and FLAIR images in axial, sagittal and coronal planes) for workup of VSs.
|
III
|
The sensitivity of MRI for correctly diagnosing VSs was 100% and specificity was 92.86% with a positive predictive value of 94.12% and accuracy of 96.67%.
|
|
Tomagane et al, 2013
|
Retrospective study of the ability of PRESTO MRI sequence to distinguish between VSs (12 patients) and meningiomas (12 patients).
|
III
|
MRI with PRESTO sequence and CT were performed. PRESTO imaging showed schwannomas exhibit intratumoral spotty signal voids significantly more frequently than meningiomas, which correspond to microhemorrhages or hemosiderin deposits histologically.
|
|
Haque et al, 2011
|
Prospective study of 42 consecutive patients referred for the evaluation of VSs.
|
II
|
After complete MRI evaluation, 61.9% were diagnosed with VSs. MRI provided 96% sensitivity, 88.2% specificity, 92.3% PPV, 93.75% NPV, and 92.86% accuracy in diagnosis of VS. (Class II achieved through comparison of MRI findings with gold standard operative histology in prospective fashion.)
|
|
Bhadelia et al 2008
|
Retrospective study of cochlear FLAIR signal in 15 patients with VSs compared to 25 age-matched controls.
|
III
|
Patients with VSs have increased cochlear FLAIR signal intensity on the affected side compared with the unaffected side and healthy subjects. 8/15 patients also showed increased signal intensity in other portions of the membranous labyrinth, such as the semicircular canals and vestibule on the affected side.
|
|
Thamburaj et al 2008
|
Prospective study of contribution of GRE imaging to differentiate VSs (n = 15 patients) from meningiomas (n = 5) based on presence of intratumoral microhemorrhages.
|
II
|
T2-weighted GRE MRI revealed microhemorrhages in 93.75% of VSs, a significantly higher rate than in meningiomas. T2 TSE and FLAIR imaging detected microhemorrhage in only 12.5% of VS cases.
(Class II achieved through comparison of MRI findings with criterion standard operative histology in prospective fashion.)
|
|
Zealley et al 2000
|
Prospective study comparing contrast-enhanced T1-weighted and FSE T2-weighted MRI in 1233 consecutive patients referred for exclusion of ANs.
|
II
|
Contrast-enhanced T1-weighted MRI was needed in addition to FSE T2-weighted imaging to confirm diagnosis in 44% of 33 cases identified to have VSs and 90% (9/10) of intracanalicular tumors. Identification of small intracanalicular VSs cannot rely on FSE T2-weighted imaging alone. (Class II achieved through comparison of FSE T2 findings with criterion standard contrast-enhanced MRI by independent radiologists in prospective fashion.)
|
|
Held et al 1999
|
Retrospective study in 20 VS patients to compare T2-weighted CISS with post-contrast T1-weighted MPRAGE imaging in diagnosis of VSs.
|
III
|
All tumors were detected by both contrast-enhanced 3D MPRAGE and 3D CISS, with diameters equally well measured. 3D CISS better defined nerve of origin in lesions ≤10 mm, but with larger tumors, neither CISS nor MPRAGE yielded nerve of origin.
|
|
Tan 1999
|
Retrospective study of FSE MRI versus CT in the diagnosis of VSs in 123 sensorineural hearing loss patients.
|
III
|
High-resolution FSE MRI is more sensitive than contrast-enhanced CT for the diagnosis of VSs.
|
|
Held et al 1997
|
Retrospective study of 42 MRIs in 38 patients scanned with enhanced and unenhanced 3D MPRAGE and 3D CISS sequences to evaluate CPA pathologies.
|
III
|
3D MPRAGE and 3D CISS are complementary MRI modalities. T1-weighted 3D MPRAGE is preferred to T1-weighted 2D spin echo sequences because of multiplanar reconstruction capabilities.
|
|
Hermans et al 1997
|
Retrospective study of contrast-enhanced T1-weighted and T2-weighted CISS MRIs in 83 patients imaged to rule out VSs, with diagnosis of 18 VS tumors after review of imaging by 2 radiologists.
|
III
|
CISS and T1 postcontrast MRI offered high sensitivity (89–94%), specificity (94–97%), and accuracy (94–95%) in detecting VSs, with slight limitation for small intracanalicular and intralabyrinthine tumors. Variations in intraobserver (kappa 0.93–1) and interobserver (kappa 0.83–0.84) reproducibility were low. (Class II achieved through independent comparison of CISS MRI findings with criterion standard contrast-enhanced MRI.)
|
|
Soulie et al 1997
|
Prospective study comparing the detection of VSs using T2-weighted FSE MRI vs contrast-enhanced T1-weighted MRI in 110 patients referred for suspected retrocochlear pathology based on sensorineural hearing loss, vertigo, or both.
|
II
|
Diagnosis of VSs can be excluded with normal 2D-FSE T2-weighted MRI with no additional gadolinium-enhanced T1 sequence. However, 6 false positives were identified relying on 2D-FSE, as confirmed by contrast-enhanced T1 MRI, resulting in an overall 100% sensitivity and 93% specificity for T2-weighted FSE imaging in ruling out VSs. (Class II achieved through comparison of FSE findings with criterion standard contrast-enhanced MRI by independent radiologists in prospective fashion.)
|
|
Stuckey et al 1996
|
Prospective study comparing accuracy of CISS MRI with contrast-enhanced T1-weighted spin-echo MRI in detecting VSs in 125 consecutive patients after review by 2 observers.
|
II
|
Across 18 cases of CPA or IAC pathologies detected by T1-weighted MRI, CISS revealed a sensitivity of 94-100% and a specificity of 94-98% for radiographic detection of tumor. (Class II achieved through comparison of CISS findings with criterion standard contrast-enhanced MRI by independent radiologists in prospective fashion.)
|
AN, acoustic neuroma; CISS, constructive interference in steady state; CPA, cerebellopontine angle; DWI, diffusion weighed imaging; FLAIR, fluid attenuated inversion recovery; GRE, gradient echo; IAC, internal auditory canal; MRI, magnetic resonance imaging; MPRAGE, magnetization prepared rapid acquisition gradient echo; PRESTO, principles of echo-shifting with a train of observations; TSE, turbo spin echo; VS, vestibular schwannoma.
Table 3. Magnetic resonance imaging assessment of vestibular schwannomas: Postoperative surveillance
|
Author, Year
|
Description of Study
|
Data Class
|
Conclusions
|
|
Carlson et al, 2012
|
Retrospective study of 203 patients undergoing microsurgical VS resection.
|
III
|
In 200/203 (98.5%) study patients, MRI demonstrated some postoperative enhancement within the preoperative tumor margin. During the course of follow-up, 49 (24.5%) patients demonstrated stable enhancement, 132 (66%) displayed lesion regression, and 7 (3.5%) had complete resolution of enhancement. 12 patients (5.9%) were diagnosed with recurrence at a mean of 3.0 years (median, 2.5; range 0.6–6.7) after surgery.
|
|
Carlson et al, 2011
|
Retrospective study of 80 patients who underwent VS resection and had postsurgical enhancement in the distal aspect of the IAC lateral preoperative tumor margin.
|
III
|
Nodular enhancement within the fundus of the IAC lateral to the preoperative radiological tumor margin was seen in 16/80 (20%) after gross-total VS resection. Enhancement was nodular (13/16, 81%) or thin linear (3/16, 19%).
|
|
Rampp et al, 2011
|
Prospective study of 21 patients with nontreated VSs to assess pattern of tumor enhancement on MRI at different time points after contrast administration.
|
III
|
While the appearance of overall tumor size remains stable, the interior appearance of VSs varies dependent on timing of image acquisition after contrast administration. The volume of central NEA at 11.5 minutes was only 11% of NEA at 1.5 minutes. So-called “necrotic tumor areas” may be falsely detected because of timing of image acquisition with respect to the administration of contrast medium.
|
|
Ozgen et al, 2009
|
Retrospective study of 50 MRIs in 18 VS patients.
|
III
|
There is no difference in the detection of progression by using the CISS sequence alone compared with postcontrast sequences. However, the CISS has a low sensitivity for the detection of changes in the internal architecture, which may limit its use for the follow-up of patients after radiation treatment.
|
|
Bennett et al, 2008
|
Retrospective study of 359 patients following resection.
|
III
|
299 patients had MRI imaging at 1 and 5 years for analysis and a 5-year follow-up examination. Of these patients, 284 were found to have no enhancement at both one and five years. Linear enhancement was seen in 10 patients but did not enlarge in any patient. Nodular enhancement of the IAC was observed in three patients. Two patients with nodular enhancement had tumor recurrence.
|
|
Brors et al, 2003
|
Retrospective study of postoperative MRIs of 70 patients who underwent resection of a unilateral VS.
|
III
|
At 3–6 months, all patients showed IAC enhancement from faint to high intensity; none had mass-like enhancement. At 12–24 months, 30/70 (42%) had decreasing, 35/70 (50%) stable, and 5/70 (8%) increasing enhancement; all 5 enhancers had intense nodular or mass-like pattern. At 36–48 months, 28/45 (59%) showed decreasing, 12/45 (29%) stable, and 4/45 (11%), with enhancers again nodular. At 4–6 years, 12/19 (63%) had decreased; 2/19 (11%) had no change, and 5/19 (26%) still increasing nodular or mass-like enhancement (proven in all 5 pathologically).
|
|
Umezu et al, 1999
|
Retrospective study of 22 VS patients with 56 MRI examinations obtained between days 1 and 930 after surgery, to examine the pattern and timing of postoperative contrast enhancement after VS resection.
|
III
|
MRI should be obtained within the first 2 days after surgery, to minimize the confounding enhancement of muscle or fibrin glue packing after VS surgery.
|
|
Kremer et al, 1998
|
Prospective study of 21 patients who underwent 3 MRI examinations after VS resection.
|
III
|
On early imaging (within 3 days postoperatively), no residual tumor but linear enhancement was seen in 16/21 (77%). On intermediate scans (6 weeks), nodular enhancement was seen in 16/18 (89%) of patients in whom muscle graft and fibrin glue were used. On late examination (6 months), nodular enhancement remained in 13/18 (72%) of implant patients, but was diminished in 10/13 compared to the intermediate scan.
|
|
Weissman et al, 1997
|
Retrospective study of postoperative MRI patterns in 36 patients following VS resection.
|
III
|
Linear enhancement in the IAC is probably normal after surgery. Nodular and mass-like enhancement and any progressive enhancement may require close follow-up to monitor growth of residual tumor. Labyrinthine hyperintensity may reflect blood metabolites. VS patients should be followed by MRI years after surgery.
|
|
Mazzoni et al, 1996
|
Retrospective study of postoperative MRI in 104 consecutive patients undergoing hearing preservation surgery for VSs.
|
III
|
Postoperative MRI should be performed at 1 and 3 years after surgery, and possibly at 5 and 8 years (speculative). Rounded nodular enhancing area reflected tumor, linear enhancement did not.
|
CISS, constructive interference in steady state; IAC, internal auditory canal; MRI, magnetic resonance imaging; NEA, nonenhancing area; VS, vestibular schwannoma.
Table 4. Magnetic resonance imaging facial nerve assessment
|
Author, Year
|
Description of Study
|
Data Class
|
Conclusions
|
|
Choi et al, 2014
|
FN course in 11 patients with VSs was compared between preoperative DTT and intraoperative observation in prospective fashion. FN course was confirmed at 3 months postoperatively on tractography and function assessed on mean follow-up of 20 months.
|
III
|
Preoperative tractography prediction of FN course correlated with intraoperative findings in all (100%) cases.
|
|
Nakai et al, 2013
|
FN course in 82 patients with VSs was compared between preoperative contrast-enhanced balanced fast field echo MRI and intraoperative observation in prospective fashion.
|
III
|
FN was identified in 46.3% (38/82) of patients using contrast-enhanced MRI, of which 74% (28/38) demonstrated congruence between preoperative predicted course and intraoperative observation. FN was more likely to be visualized in smaller tumors, with a solid consistency.
|
|
Zhang et al, 2013
|
FN course was identified preoperatively using DTT in 8 patients with large (≥30-mm diameter) VSs and compared to intraoperative observation and EMG retrospectively.
|
III
|
FN could be identified in 87.5% (7/8) of cases preoperatively using DTT, and agreed to intraoperative evaluation in all of these cases. FN was anatomically preserved with HB grade I–II function in all cases.
|
|
Chen et al, 2011
|
The relationship of the facial, trigeminal, and abducens nerves juxtaposed to tumor were reconstructed using DTI tractography superimposed on 3D tumor volumes in 3 patients with vestibular schwannoma.
|
III
|
The facial/cochlear-vestibular complex could be reconstructed in all (3/3) cases, but individual contributions of facial versus vestibular nerves within the complex could not be distinguished, nor could cisternal segment fibers in 1 case of a smaller tumor. Trigeminal nerve could be reconstructed with ease in all cases.
|
|
Gerganov et al, 2011
|
22 patients with large VSs and normal baseline facial function underwent preoperative DTI and CISS imaging for prospective determination of the course of the cisternal FN segment, in relation to tumor, which was correlated with intraoperative observations of the FN position. Surgeon was blinded to results of fiber tracking. The morphologic shape of FN was also recorded as flat or compact, based on intraoperative observation.
|
III
|
Tractography predicted FN cisternal (CPA) segment course and displacement by tumor in 90.9% (20/22) of cases compared to intraoperative evaluation. No DTI fiber patterns were found to be correlated with either flat or compact FN morphology.
|
|
Liang et al, 2010
|
The ability of T2-weighted CISS versus TSE imaging to identify facial and vestibulocochlear nerves was compared in 48 volunteer subjects, as rated by 2 observers. CISS alone was performed in 8 patients with cerebellopontine angle pathology (1 bilateral VS).
|
III
|
CISS images were significantly better than TSE for visualizing canalicular segments of facial and vestibulocochlear nerves and slightly better for the cisternal segments of facial and vestibulocochlear nerves.
|
|
Taoka et al, 2006
|
FN course in 8 patients with VSs was compared between preoperative DTI reconstruction, T2-weighted MR cisternography, and intraoperative observation.
|
III
|
FN course was reconstructed using tractography in 87.5% (7/8) of cases preoperatively and corresponded to intraoperative observation of the nerve trajectory in 71.4% (5/7). DTI tract was not obtained on the smaller tumor (18 mm diameter), which was the only case where preoperative magnetic resonance cisternography could identify the FN.
|
|
Kocaoglu et al, 2003
|
Facial and cochlear nerve course were prospectively identified in 22 patients with small VSs undergoing hearing-preservation operations using contrast-enhanced T1-weighted and CISS MRI sequences by 2 independent radiologists.
|
III
|
Spatial relationship of FN and tumor could be determined in 82% (18/22) of cases on CISS images, but not on any of the contrast-enhanced T1-weighted sequences. No correlation between the direction of FN displacement and postoperative facial palsy or hearing loss was observed.
|
|
Sartoretti-Schefer et al, 2000
|
Relationship of FN and tumor investigated in 22 patients with VSs using T2-weighted fast spin echo and T1-weighted MRI.
|
III
|
FN relation with the VS could be discerned in 86% of cases using T2-weighted FSE imaging but not T1-weighted sequences, with diminishing visualization of FN in larger sized tumors.
|
|
Schmalbrock et al, 1999
|
21 patients with 27 VSs (81% ≤1 cm3) were retrospectively assessed with T2-weighted SIMCAST and T1-weighted contrast-enhanced MRI for assessment of tumor appearance and its relation with the FN.
|
III
|
Facial or vestibulocochlear nerve branches were seen in 63% (17/27) of affected ears on axial T2-weighted SIMCAST imaging. SIMCAST delineated the relation of the 7/8 nerves and tumor with greater clarity than T1 sequence, while T1 contrast-enhanced sequence delineated the tumor-brain boundary better. Both imaging modalities were consistent in demarcating VS size.
|
|
Shigematsu et al, 1999
|
7th and 8th nerves and their relation to the tumor was prospectively evaluated in a phantom model and in 9 patients with 11 VSs using precontrast and contrast-enhanced CISS by 2 neuroradiologists. Imaging predictions were not compared to intraoperative observations in the 7 patients who underwent resection.
|
III
|
Addition of contrast to CISS increased the discrimination between cranial nerve and solid enhancing tumor and in identifying the 7th and 8th nerves at the IAC.
|
|
Jung et al, 1998
|
Retrospective analysis of the correlation between preoperative prediction of FN displacement based on contrast-enhanced T1-weighted and T2-weighted MRI and intraoperative observation in 19 patients with extra-large (>40 mm in extrameatal diameter) VSs. The likely course of the FN was extrapolated from the relationship between the intrameatal and extrameatal axes of the tumor mass on MRI.
|
III
|
FN displacement was predicted in 80% of cases based on the angles formed between the intrameatal tumor and extrameatal tumor in relation to the IAC.
|
|
Casselman et al, 1993
|
Retrospective series assessing the course of the facial, cochlear, superior vestibular, and inferior vestibular nerves in 50 normal ears and 10 ears with pathology (3 with VSs) using CISS MRI.
|
III
|
FN was identified in the IAC in 90% of normal ears in axial and coronal CISS sequences, most easily in the cisternal and horizontal segments, and least reliably around the posterior genu and vertical segment, where sparse CSF surrounds the nerve.
|
HB, House–Brackmann; CISS, constructive interference in steady state; CPA, cerebellopontine angle; CSF, cerebrospinal fluid; DTI, diffusion tensor imaging; DTT, diffusion tensor tractography; EMG, electromyography; IAC, internal auditory canal; MRI, magnetic resonance imaging; MPRAGE, magnetization prepared rapid acquisition gradient echo; SIMCAST, segment-interleaved motion-compensated acquisition in steady state; TSE, turbo spin echo; VS, vestibular schwannoma.
Table 5. Use of magnetic resonance imaging for tumor surveillance
|
Author, Year
|
Description of Study
|
Data Class
|
Conclusions
|
|
Fayad et al, 2014
|
Retrospective review of 114 patients with presumed VSs undergoing conservative management. Patients were followed for a mean duration of 4.8 years and with annotation of growth, initiation of treatment, and audiologic measures. Mean tumor diameter of 10.5 mm. Growth was defined as ≥2 mm change in the maximal tumor diameter. This is a longer-term follow-up from Fucci et al, 1999.
|
III
|
37.7% of tumors had grown at 5 years. 51.7% patients whose tumors had grown demonstrated growth at 1 year after initial MRI. After 5 years of no growth, 4% of patients showed growth. Monitoring by MRI is typically scheduled 6 months after the initial visit, at 1 and 2 years after that, at 5 years, and then if symptoms change.
|
|
Moffat et al, 2012
|
381 patients were included who were managed conservatively and who had at least 2 MRI scans to assess growth and associated predictive features. Mean interval between first and last scans was 4.2 years (0.5–17 years).
|
III
|
59.3% of tumors did not grow over 5 years. Over half of growing tumors grew within the first 18 months after diagnosis. 7% of tumors grew after 5 years. The most common growth pattern was progressive growth, followed a pattern of no growth followed by growth. The authors recommend an MRI 6 months after initial diagnosis followed by annual scans, at which point scans can be done every 2 years for 6 years and then every 3 years.
|
|
Varughese et al, 2012
|
Prospective study of 178 patients with VSs <2 cm over a mean follow-up period of 35.4 months to assess growth rate, features predictive of growth, and which measurement method was most accurate. They measured using diameter, volume, and VDT.
|
III
|
Three measurement modalities were used. Using the single diameter method, 29.2% tumors grew >1 mm. A VDT of 5.22 years strongly separated growing from nongrowing tumors. Patients are scanned at 12, 24, and 60 months for those being managed conservatively.
|
|
Suryanarayanan et al, 2010
|
327 patients with sporadic VSs were observed and MRI measurements taken with a mean follow-up of 3.6 years (range 1–14 years) to assess growth rate and associated predictive features.
|
III
|
Data on growth were available for 240 patients. 68% were stable. All growth began within the first 4 years of follow-up. Tumors >15 mm cisternally were likely to continue growing. The average growth rate overall was 1.1 mm/year.
|
|
Artz et al, 2009
|
Retrospective review of a prospectively gathered database comprising 234 sporadic VSs managed conservatively for a mean of 28 months (range 4–120 months) to ascertain predictors of growth
|
III
|
Symptoms and anatomy of VSs at initial presentation help to predict future growth. “High-risk” tumors were either extrameatal with short duration of hearing loss and either unsteadiness/vertigo or no SSHL, or were intrameatal with short duration of hearing loss, unsteadiness/vertigo, and no SSHL. In this group, the risk of growth was 36.9% in the first year and 64.6% in 2 years. Low-risk tumors were extrameatal with no other risk factors or intrameatal with at most one other risk factor. In this group of tumors, the risk of growth was 2.5% in the first year and 12.7% within the first 2 years.
|
|
Bakkouri et al, 2009
|
Retrospective review of 325 patients with VSs managed conservatively with at least 1 follow-up scan. The authors described growth rates. Follow-up ranged from 1–9 years.
|
III
|
The overall mean tumor growth rate was 1.15 mm/year. The mean percentages of cases that did not show any tumor growth, tumor growth <3 mm/year, and growth ≥3 mm/year during all observation periods were 57.8%, 28.9%, and 12%, respectively. Slowly progressive symptoms correlated with slower growth.
|
|
Martin et al, 2009
|
Retrospective consideration of 276 patients with ≥1 follow-up scan followed over a mean period of 43 months to determine growth rate.
|
III
|
Of 276 patients, 62 (22%) demonstrated growth. Of the growing tumors, 65% grew slowly and 35% grew more rapidly; most rapidly growing tumors had grown even at 6-month MRI. Of all growing tumors, 90% were detected within 3 years. They recommend an initial MRI scan at 6 months, with annual scans for 2 years followed by a scan 2 years later, then every 5 years.
|
|
Ferri et al, 2008
|
Retrospective review of 124 patients managed conservatively for an average follow-up period of 4.8 years to establish growth patterns.
|
III
|
64.5% of tumors showed no increase in size (defined by change >2 mm). Of growing tumors, 45.4% grew within the first year; 22.7% grew at least 3 years after initial scan. No growth occurred after 6 years. The authors scan 6 months after initial scan, then at 12-month intervals unless growth is noted, which prompts a 6-month scan if no intervention occurs.
|
|
Solares et al, 2008
|
Retrospective review of a prospectively collected database; 110 patients with sporadic VSs with at least 2 interval MRI scans were followed for a mean of 31 months (range 6–154 months) to assess growth patterns.
|
III
|
The 5-year no growth rate was 70.6% overall. Intracanalicular tumors were much less likely to grow (89.8% NGR vs 73.9% and 45.2% for grade I and II tumors, respectively). 10% of tumors regressed. Patients had an MRI 6 months after tumor discovery, then yearly if stable.
|
|
Stangerup et al, 2006
|
Retrospective review of 552 patients with at least 2 MRI scans managed conservatively to determine growth rates; mean observation period was 3.6 years (range 1–15 years). Growth to extrameatal extension was the definition for growth in intrameatal tumors. In extrameatal tumors, an increase of >2 mm in the largest extrameatal diameter was defined as growth, and a decrease of >2 mm was defined as shrinkage.
|
III
|
Overall, 29% of extrameatal tumors grew. Of growing tumors, most began growth in the first year; the sooner the growth detection, the faster the growth rate. No tumor growth was initiated after the fourth year of observation. Of intrameatal tumors, 17% grew, with the majority growing in the first year, with no growth after the fourth year. The authors recommended yearly MRI for 5 years; every other year for 4 years; and a final MRI 5 years after that.
|
|
Bozorg Grayeli et al, 2005
|
Retrospective review of 111 patients with intracanalicular or grade 2 VSs managed conservatively with serial MRI scans and mean follow-up of 33 months (range 6–111 months) with a view to establishing growth rates.
|
III
|
Growth was defined as a ≥2-mm increase in maximum AP size. 47% of tumors were stable; 47% increased in size; and 6% decreased in size. The mean growth rate was 1.1 mm/year.
|
|
Flint et al, 2005
|
Retrospective review of 102 patients with unilateral VSs <24 mm followed for a median of 25.5 months to assess growth rates.
|
III
|
62% of tumors showed no growth; 2 patients had tumor regression. 80% of patients with growing tumors had growth in the first year. Of these, 66% continued to grow. 20% of growing tumors had a latency period of no growth (range 8–60 months). Increase in size in first year may predict future growth.
|
|
Hoistad et al, 2001
|
Retrospective review of 102 patients with unilateral VSs followed for a mean of 28.5 months (range 6 months–10 years) to assess tumor growth.
|
III
|
53% of patients showed no growth; 44% grew ≥1 mm, with the mean growth rate of growing tumors estimated at 2 mm/year. Conservative management is reasonable with continued follow-up.
|
|
Tschudi et al, 2000
|
Retrospective review of 74 patients followed for a mean of 35 months to establish growth rates.
|
III
|
68.9% of tumors did not grow; 16% regressed. Of growing tumors, growth in the first year was predictive of future growth, as were the presence of the following symptoms: tinnitus, sudden hearing loss, or dizziness.
|
|
Fucci et al, 1999
|
Retrospective review of 119 patients with presumed vestibular schwannomas undergoing conservative management. Patients were followed for a mean duration of 2.5 years (range 5 months–8 years) and growth, initiation of treatment, and audiologic measures were noted. At least 2 MRIs were performed per patient.
|
III
|
66% of tumors did not grow during the study period. While the overall growth rate was 1.2 mm/year, growing tumors grew 3.8 mm/year on average. Size >20 mm at presentation predicted future growth. The authors recommend the initial follow-up scan occur at 6 months.
|
MRI, magnetic resonance imaging; NGR, no growth rate; SSHL, sudden sensorineural hearing loss; VDT, volume doubling time; VS, vestibular schwannoma.
Table 6. Cystic vestibular schwannoma behavior
|
Author, Year
|
Description of Study
|
Data Class
|
Conclusions
|
|
Dunn et al, 2014
|
52 patients with VSs with little or no extension into the IAC were retrospectively reviewed with regard to surgical detail and clinical outcome.
|
III
|
Medial VSs demonstrated a trend to increased adherence to the brainstem and FN. 71% of medial VSs were cystic. The FN outcomes were grade I/II in 87% of cases at latest follow-up, with 53% of patients experiencing transient FN palsy.
|
|
Metwali et al, 2014
|
37 patients with cystic VSs were reviewed and compared to similarly sized non-cystic tumors with respect to FN outcome postoperatively and at 1 year.
|
III
|
Cystic tumors tended to be large overall (T4 classification). Higher rates of incomplete resection were noted in cystic cases, as were lower rates of unfavorable FN outcome early after surgery (37.8%), when compared to solid tumors (17.5%). These rates equilibrated at 1 year (8.1% vs 6.2%). There were higher rates of postoperative hematoma and hydrocephalus in cystic cases.
|
|
Yashar et al, 2012
|
Retrospective review of 23 patients undergoing surgery for cystic VSs.
|
III
|
Cystic schwannomas represented 12.8% of their overall series. Complete resection rates in these tumors was 48%. HB I–III function was achieved in 73% of cystic tumors in which a complete resection was achieved.
|
|
Piccirillo et al, 2009
|
Retrospective review of morbidity and FN outcome in 57 cystic VSs with at least 1-year follow-up compared with results in patients with solid tumors.
|
III
|
Complete resection rates were similar in cystic vs solid tumors (84% vs 82%). The authors classified cysts as thick-walled and central (type A, with subtypes) or thin-walled and peripheral (type B, with subtypes). Overall, 1-year FN outcomes were similar between both groups (81% grade I-III). However, the authors recommend subtotal resection in patients with type B cystic schwannomas in their classification scheme.
|
|
Mehrotra et al, 2008
|
Retrospective review of clinical outcomes of 22 patients with giant (>4 cm) cystic VSs with results compared to solid tumors of matched size (n = 40).
|
III
|
Patients with cystic tumors were likely to experience rapid deterioration. A large cystic component made surgery more challenging; overall, early FN outcomes were superior in patients with cystic tumors, though longer follow-up data are not presented.
|
|
Sinha et al, 2008
|
Retrospective review of 58 patients with cystic VSs comparing surgical outcomes with patients with solid tumors.
|
III
|
Cystic schwannomas were less likely to be completely resected than solid tumors, and were more likely to have unfavorable FN outcomes.
|
|
Jones et al, 2007
|
Retrospective review of 70 patients with cystic VSs and matched cohort of patients with solid tumors to assess effect of cystic nature on FN outcome at 2 years.
|
III
|
At 2-year follow-up, FN function is not statistically different between a group of patients with cystic and solid tumors. However, there is a trend to lower rates of grade I function and higher rates of grade VI function in the cystic tumor cohort.
|
|
Benech et al, 2005
|
Retrospective review of 80 larger VSs, of which 26 were cystic, with a view to comparing presentation and surgical outcomes.
|
III
|
Cystic tumors were associated with shorter duration of symptoms; complete resection rates were similar. Favorable FN outcomes (HB I–III) were 58% at 1 year.
|
|
Wandong et al, 2005
|
Retrospective review of a series of vestibular schwannoma patients to define the incidence and clinical characteristics of cystic tumors; 22 cystic schwannomas had surgery in this series
|
III
|
Cystic schwannomas constituted 7.3% of their series; the rate of FN continuity was 86%, and HB IV–VI at 2 years = 41%. They report worse outcomes in their cohort of cystic schwannoma patients.
|
|
Zaouche et al, 2005
|
Retrospective review of 424 patients undergoing surgery for vestibular schwannomas to identify factors predicting facial palsy in the immediate postoperative period.
|
III
|
A heterogeneous and cystic appearance on preoperative MRI was associated with a statistically significant increased likelihood of HB ≥3 within 10 days of surgery
|
|
Fundova et al, 2000
|
Retrospective review of the surgical outcomes of 44 cystic VSs (5.7% of their overall series) compared with 151 solid tumors.
|
III
|
Resection rates were similar in the cystic and solid groups. FN outcome analysis notable for a statistically significant increase in patients with grade VI facial function in cystic cases when compared to solid tumors (41% vs 27%) at 1 year. While the authors observed less structural adherence of tumors to surrounding tissue in cystic cases, the complication rates in these cases were higher when compared with patients with solid tumors (31.5% vs 20%). Average tumor size was 3.9 cm.
|
|
Shirato et al, 2000
|
Retrospective review comparing the results and complications of SRT in 20 cystic schwannomas compared to 45 solid schwannomas. The mean follow-up period was 37 months. Overall tumor control was defined as no tumor growth of >2 mm after 2 years or no requirement of salvage surgery.
|
III
|
The 3-year tumor reduction rate was 31% for solid tumors and 93% for cystic tumors, with the difference being significant. However, cystic tumors tended to enlarge in the posttreatment period prior to size reduction. The authors report that fractionated SRT is safe in these tumors with size <3 cm, in contrast to previous reports on high-dose SRS in cystic tumors.
|
|
Samii et al, 1997
|
Retrospective review of 1000 VS resections via the suboccipital approach to identify results and complications.
|
III
|
In cases of cystic tumor formation, the anatomic preservation rate of the FN was reduced from 93% to 88% and of the cochlear nerve from 68% to 55%. Cystic VSs were also more likely to hemorrhage postoperatively.
|
|
Charabi et al, 1994
|
23 cystic VSs were retrospectively reviewed to annotate imaging characteristics and growth patterns.
|
III
|
The overall incidence of cystic schwannomas was 4%. In 4 patients, growth rates between 6 months to 1 year was from 2–29 mm, all from cystic expansion. The authors concluded that patients with cystic tumors are at higher risk for symptomatic worsening given their higher growth rates, and a “wait and see” philosophy should not be adopted.
|
|
Charabi et al, 1994
|
Retrospective review of surgical outcomes of 23 cystic schwannomas with attention to FN outcome and rates of resection.
|
III
|
Complete resection was achieved in 91% of cases, in which tumors were a mean of 4.5 cm. At 1 year, 65% of patients had unfavorable FN function (grade IV–VI). The authors recommend a lower threshold for leaving residual cyst wall if the exact anatomy is unclear.
|
|
Tali et al, 1993
|
Retrospective review of 16 cases of cystic schwannoma to document the MRI appearance of these lesions; the incidence of this subgroup was determined in a series of 411 cases.
|
III
|
The incidence of cystic schwannomas was 19.3%. They generally displayed high signal intensity on T1 and T2 sequences, with peripheral enhancement.
|
|
Wallace et al, 1993
|
Retrospective review of incidence of cystic tumors in 35 patients with VSs.
|
III
|
Cystic schwannomas, defined by the cystic component occupying at least 50% of the tumor mass, accounted for 24% of VSs in this series.
|
FN, facial nerve; HB, House–Brackmann; IAC, internal auditory canal; MRI, magnetic resonance imaging; VS, vestibular schwannoma.
Table 7. Impact of lateral internal auditory canal involvement
|
Author, Year
|
Description of Study
|
Data Class
|
Conclusions
|
|
Gerganov et al, 2009
|
Retrospective review of 99 consecutive VS cases resected by retrosigmoid approach by single surgeon, evaluating the relationship of tumor size, intrameatal tumor extension pattern, and bony IAC involvement with preoperative hearing function.
|
III
|
The degree of intrameatal tumor growth was significantly correlated with the level of preoperative hearing. The distance between the lateral tumor end and the fundus showed significant correlation with shorter distance associated with worse Hanover score.
|
|
Gerganov et al, 2009
|
Retrospective analysis of 99 consecutive cases resected by retrosigmoid approach by single surgeon, evaluating hearing preservation based on tumor extension pattern, volume, diameter, shape, and bony IAC involvement.
|
III
|
Increasing tumor volume, extrameatal tumor volume, and stage significantly correlated with worse postoperative HB grade. Intrameatal tumor length, intrameatal tumor width, tumor-fundus length showed no impact on immediate postoperative HB grade.
|
|
Mohr et al, 2005
|
Retrospective study of 128 cases using intraoperative monitoring and following a retrosigmoid approach. The maximal extrameatal size of the tumor, its extension within the IAC, and pre- and postoperative hearing quality, according to the Gardner–Robertson classification, were evaluated.
|
III
|
With regard to filling of the IAC, among 63 patients harboring a tumor 15 mm or smaller in whom MRI was available, hearing was preserved more frequently in patients with partial filling (52.8% of 36) than those with complete filling (25.9% of 27). Both tumor size and the extent of IAC filling proved statistically significant in a multivariate analysis.
|
|
Kobayashi et al, 2002
|
Retrospective study of 45 patients with small VSs resected by middle cranial fossa approach. All patients initially had full FN function on HB scale. Shortest distance and longest distance from fundus were measured on MRI.
|
III
|
Distance from fundus to AN had no effect on outcome of FN function at 2 weeks or at 3 months after surgery. Further, no correlation was observed between tumor diameter and FN function.
|
|
Rompaey et al, 2001
|
Retrospective review of 123 consecutive patients with normal FN function prior to surgical resection of VS. HB grade calculated at 1 month and 1 year after surgery was correlated with fundus obliteration on preoperative MRI.
|
III
|
One-month postoperative HB grade III or greater occurred in 29.7% of patients with complete fundal obliteration compared to 13% with no fundus obliteration (P = .04). At 1 year, HB grade III or greater occurred in 18.7% of patients with complete fundal obliteration compared to 8.6% with no fundus obliteration though statistical significance was not seen.
|
|
Matthies et al, 1997
|
Retrospective review of 202 consecutive patients evaluating preoperative high-resolution CT for specific radiographic features.
|
III
|
The length of the posterior auditory canal wall and the interear difference of the maximum porus width both correlate with the degree of preoperative hearing deterioration. The extent of the widening of the IAC is of predictive importance for the chances of postoperative hearing preservation or hearing loss. The extent of tumor growth anterior and caudal to the IAC in large tumors is of significant importance for prediction of postoperative hearing function. The tumor extension in all directions and the extent of cystic tumor components correlate with the pre‐ and postoperative function of the facial and cochlear nerves.
|
AN, acoustic neuroma; CT, computed tomography; FN, facial nerve; HB, House-Brackmann; IAC, internal auditory canal; MRI, magnetic resonance imaging; VS, vestibular schwannoma.
Table 8. Magnetic resonance imaging of vestibular schwannomas associated with neurofibromatosis type 2
|
Author, Year
|
Description of Study
|
Data Class
|
Conclusions
|
|
Mallory et al, 2014
|
26 patients with 32 NF2-related VSs were retrospectively evaluated for tumor control and hearing outcomes after SRS.
|
III
|
Improved tumor control in NF2 VSs was associated with higher marginal doses than commonly prescribed for sporadic VSs. Hearing outcomes were poor even with reduced marginal doses. Anatomic preservation of cochlear nerve with SRS may permit cochlear implantation and hearing rehabilitation.
|
|
Dombi et al, 2013
|
Proposed consensus guidelines from the Tumor Measurement Working Group of the Response Evaluation in Neurofibromatosis and Schwannomatosis committee for the evaluation of imaging response in clinical trials for NF-related tumors.
|
III
|
Volumetric analysis of MRI is recommended to sensitively and reproducibly evaluate changes in tumor size, with a 20% volume change considered to be a decrease or increase in tumor size. Standardization of criteria enables meaningful comparison across clinical trials.
|
|
Goutagny et al, 2013
|
7 NF2 patients diagnosed at ≥70 years of age were reviewed for genetic profile, tumor growth curves, and clinical course.
|
III
|
4/7 patients harbored bilateral VSs and 3/7 unilateral VSs, with no significant growth in 72% (8/11 tumors) over a mean follow-up of 96 months. Absence of germline NF2 mutation suggested a high prevalence of NF2 somatic mosaicism in these older patients. Long-term stability among most lesions suggests observation as initial management policy.
|
|
Dirks et al, 2012
|
The growth patterns of intracranial tumors were retrospectively analyzed in 17 NF2 patients with a minimum of 4 years of clinical and MRI follow-up.
|
III
|
NF2-associated intracranial tumors most frequently demonstrate a saltatory growth pattern. Because of the lifetime risk for tumorigenesis and the unpredictable nature of radiographic progression and symptom onset in NF2 patients, resection should be reserved for symptom-producing tumors. Establishing the efficacy of nonsurgical therapeutic interventions must be based on long-term follow-up (several years).
|
|
Peyre et al, 2011
|
11 NF2 patients treated for bilateral VSs were retrospectively reviewed to assess changes in size of the contralateral VS after one side is resected in long-term follow-up (mean 7.6 years).
|
III
|
The velocity of diametric expansion is significantly elevated after resection of a contralateral VS (4.4 ± 3.4 mm/year) compared to before (2.5 ± 2.2 mm/year). The growth patterns of both VSs were similar in 9/11 cases before surgery. Increased postoperative growth was associated with decreased hearing in 3 cases. Removal of a VS in NF2 patients may precipitate an increase in the growth rate of the contralateral VS.
|
|
Fisher et al, 2009
|
52 patients with bilateral VSs were retrospectively reviewed for tumor progression and hearing function.
|
III
|
Tumor size increased and hearing decreased in a 1-year period. However, changes in status on one side cannot be used to predict changes in the other side.
|
|
Ito et al, 2009
|
Retrospective review of 27 NF2 patients with 54 VSs followed for a mean of 86 months to identify factors predicting further growth of bilateral VSs.
|
III
|
Among an assessment of a variety of features (including age at onset, gender, coexistence of other tumors and volume indices), only age of onset correlated with growth (and only in posttreatment course, P = .007). Pattern suggests that after treatment particularly close follow-up may be warranted for patients with onset at an early age.
|
|
Harris et al, 2008
|
Retrospective study of 10 NF2 patients with 43 MRI studies.
|
III
|
Linear measurements underestimate VS growth rate compared with volumetric measures in NF2 patients.
|
|
Slattery et al, 2005
|
Retrospective review of prospective comparison of concordance in VS measurements across 115 MRIs from 57 NF2 patients between local radiologists and experienced neuroradiologists.
|
II
|
Fair concordance was observed between local radiologist and experienced neuroradiologist assessments. Least variability was found in thin slice postcontrast studies in patients without previous surgery. Neuroradiologist measurements were superior in postoperative tumors and tumors <5 mm. Strategies for uniform reporting are proposed, including use of fat suppression for posttreatment scans, thin-slice no skip postcontrast scans, and measurement of greatest overall diameter in addition to conventional measurement angles. (Class II achieved through blinded comparison of MRI assessments among radiologists in a prospective fashion.)
|
|
Slattery et al, 2004
|
Retrospective assessment of changes in VS size in patients enrolled in NF2 Natural history study at short-term and long-term follow-up.
|
III
|
On average, VSs in NF2 patients increased 1.3 mm/year in short-term follow-up (defined as 9 months–2 years) and 1.9 mm/year on long-term follow-up (defined as 3–4 years), with 8% exhibiting growth of ≥5 mm over 4 years.
|
|
Subach et al, 1999
|
Retrospective assessment of tumor control, hearing preservation, and facial function after SRS in 40 NF2 patients with 45 tumors over a 10-year period.
|
III
|
36 months after SRS, 36% of VSs regressed, 62% remained unchanged, and 2% grew. Useful hearing was preserved in 6 (43%) of 14 patients, with greater success (67%) after modifications made in 1992; rate of hearing preservation may be better with radiosurgery than with other available techniques. Normal FN function was preserved in 25/31 (81%) patients. Normal trigeminal nerve function was preserved in 34/36 (94%) patients.
|
FN, facial nerve; MRI, magnetic resonance imaging; NF2, neurofibromatosis type 2; SRS, stereotactic radiosurgery; VS, vestibular schwannoma.
Table 9. Length of time for magnetic resonance imaging follow-up
|
Author, Year
|
Study Description
|
Data Class
|
Conclusions
|
|
Tang et al, 2014
|
Retrospective evaluation of 88 patients who underwent VS resection and had ≥2 postoperative MRI scans
|
III
|
Nodular enhancement increased risk for tumor growth. If there was growth, tumors with nodular enhancement typically showed increase in size beginning 2 years postoperatively, whereas those with linear or no enhancement were typically stable in size through 5 years. Younger age and larger preoperative tumor size were also risk factors for growth. Imaging recommendations: The authors recommend 1) obtaining a baseline MRI at 6 months postoperatively; 2) no further imaging until 5 years postoperatively for patients with linear or no enhancement on the baseline scan, unless develop new symptoms; 3) annual MRIs starting at 2 years postoperatively for patients with nodular enhancement on the baseline scan, with offer for further treatment if MRI shows an increase in enhancement of 97 mm2 in maximal axial area or >133 mm3 in volume.
|
|
Lemee et al, 2014
|
Retrospective review of 33 patients with a postsurgical VSTR after surgery. Patients had a biannual follow-up with clinical status and VSTR size assessment with MRI.
|
III
|
The postoperative facial function impairment and an initial remnant ≥1.5 cm3 were found to be significant risk factors of VS remnant progression in non-NF2 population in univariate analysis but not in multivariate analysis. If postoperative VS remnant is seen on initial MRI 3 months after surgery in a non-NF2 patient, and remnant >1.5 cm3 or postoperative FN function ≥HB grade IV, consider SRS. If these additional factors not seen, annual clinical and radiologic follow-up for 6 years, then once per 2 years.
|
|
Carlson et al, 2012
|
Retrospective study of 350 patients who underwent VS resection, with 203 meeting criteria (non-NF2, receiving ≥2 follow-up MRIs). Imaging characteristics and need for further intervention analyzed.
|
III
|
Of 203 patients, 144 received GTR, 59 STR (did not distinguish NTR). Mean number of postoperative MRIs 2.6. Among 191 patients without recurrence over mean follow-up of 3.5 years, 7 underwent additional treatment (5 SRS and 2 surgery) at median of 8 months postoperatively. 5 patients with recurrent tumor continued to undergo observation at the end of study period.
|
|
Tysome et al, 2012
|
Evaluation of 314 consecutively treated patients followed in a prospectively maintained database who underwent complete translabyrinthine excision of VSs and had follow-up imaging at 2 and 5 years after surgery.
|
III
|
All patients where MRI was reported to show no recurrence at 2 years (97% of 314) had no signs of recurrence on MRI at 5 years. 8 patients with MRI suspicious of recurrence (linear enhancement of IAC) at 2 years had no progression on MRI at 5–15 years. One patient had evidence of definite recurrence (nodular enhancement of IAC) at 2 years, and underwent radiosurgery at 8 years. The authors recommend a single MRI after complete resection of VS, with early imaging at 2 years after surgery to identify those at risk of recurrence. Patients with nodular enhancement should be considered to have recurrent disease. Linear enhancement on MRI should prompt repeat MRI at 5 years, with no further imaging needed if no progression is observed at that time.
|
|
Arlt et al, 2011
|
Retrospective review of tumor progression and FN function in 50 patients after surgical resection of VSs.
|
III
|
9/28 (32%) cases who received initial STR showed progression over a median follow-up of 52 months (median time to recurrence 44 months). 2/22 (9%) who received initial GTR showed tumor progression over a median follow-up of 50.5 months (median time to recurrence 41.5 months).
|
|
Carlson et al, 2011
|
16 patients with nodular enhancement in IAC among group of 350 who underwent microsurgery for VSs were retrospectively reviewed for tumor recurrence. Patients with incomplete resection, NF2, and fewer than 2 postoperative MRIs were excluded.
|
III
|
0 of 16 patients with nodular enhancement at IAC on initial MRI (obtained at 3.1 months postoperatively on average) developed recurrence on mean radiologic follow-up of 39.8 months.
|
|
Fukuda et al, 2011
|
Retrospective review of 74 patients after gross total (41), subtotal (25), or partial (8) resection of VSs to assess regrowth rate and time to regrowth.
|
III
|
VSs demonstrated regrowth of 2.4% (1/41 cases), 52% (13/25), and 62.5% (5/8) after GTR, STR, and PR, respectively, over a mean follow-up of 104 months (range 60–241 months). Time to regrowth ranged 6 to 76 months (median 31.9 months).
|
|
Peyre et al, 2011
|
11 NF2 patients treated for bilateral VSs were retrospectively reviewed to assess changes in size of the contralateral VS after one side is resected in long-term follow-up (mean 7.6 years).
|
III
|
In setting of bilateral vestibular schwannomas causing brainstem compression, the tumor growth rate of the remaining VS increases after surgery of the contralateral tumor. Recommend regular (every 6 months) radiologic follow-up after resection of 1 of 2 VSs in NF2.
|
|
Godefroy et al, 2009
|
Recurrence rates and long-term FN function were retrospectively reviewed in 51 patients after translabyrinthine resection of large VSs and correlated to the initial extent of resection.
|
III
|
GTR in 26%, NTR in 58%, STR in 16%. No patients showed disease progression over mean follow-up of 48 months.
|
|
Bennett et al, 2008
|
Retrospective study of enhancement patterns and tumor recurrence in 299 patients after resection of VSs, with MRI at 1 and 5 years.
|
III
|
284/299 patients had no enhancement on follow-up. Linear enhancement in 10 patients did not show progression to tumor recurrence during study interval. 3 patients had nodular enhancement with 2 of these having tumor recurrence over 1–5 years.
|
|
Schmerber et al, 2005
|
Retrospective analysis of 91 patients who underwent translabyrinthine GTR of VSs and who were followed for at least 5 years (mean 11 years).
|
III
|
None of the 91 patients experienced radiographic recurrence of VSs. A single gadolinium-enhanced MRI scan 5 years after surgery is advised in case of total removal. In case of any doubt about the quality of the tumor removal, MRI follow-up schedule within 2 years and 5 years of surgery establishes an initial baseline, with repeat MRI thereafter on clinical grounds.
|
|
Bloch et al, 2004
|
Retrospective review of recurrence rate in 79 VS patients after microsurgery without GTR (50 with NTR and 29 with STR).
|
III
|
3% recurrence observed after NTR and 32% after STR, with mean interval from surgery to detection of recurrence at 3 years (range 1–5 years).
|
|
Kameyama et al, 1996
|
Retrospective review of 11 patients who underwent STR of intracanalicular VSs, with follow-up MRI ranging from 12 to 29 years.
|
III
|
MRI of 2 patients showed no evidence of tumor remnant, 6 with small tumor in IAC, remaining 3 with intracanalicular tumor protruding slightly intracranially. None underwent reoperation. Authors conclude that small intracanalicular small remnants have low risk of progression.
|
|
Lye et al, 1992
|
14 patients with residual tumors after surgical resection of VSs were retrospectively reviewed for growth of residual tumors.
|
III
|
4/7 patients with residual tumors showed tumor enlargement on gadolinium-enhanced MRI over a mean follow-up of 70 months. An early postoperative (within 3 months) contrast-enhanced MRI is recommended in cases of suspected residual to establish baseline, repeat MRI 2 years later, and then, on clinical basis.
|
|
Kemink et al, 1991
|
Retrospective review of 20 patients who underwent microsurgery with intentional STR (8) or NTR (12). Patient characteristics, postoperative CT and MRI, and neurologic function were assessed.
|
III
|
Radiologic detectable tumor regrowth occurred in only 1 patient (with STR) over a mean follow-up of 5 years. Postoperative contrast-enhanced CT or MRI was obtained at 2 months, followed by yearly scans thereafter.
|
GTR, gross total resection; HB, House–Brackmann; IAC, internal auditory canal; MRI, magnetic resonance imaging; NF2, neurofibromatosis type 2; NTR, near total resection; PR, partial resection; SRS, stereotactic radiosurgery; STR, subtotal resection; VS, vestibular schwannoma; VSTR, vestibular schwannoma tumor remnant.
References
1. Tan TY. Non-contrast high resolution fast spin echo magnetic resonance imaging of acoustic schwannoma. Singapore Med J 1999;40(1):27-31.
2. Tomogane Y, Mori K, Izumoto S, et al. Usefulness of PRESTO magnetic resonance imaging for the differentiation of schwannoma and meningioma in the cerebellopontine angle. Neurol Med Chir (Tokyo) 2013;53(7):482-489.
3. Soulié D, Cordoliani YS, Vignaud J, Cosnard G. MR imaging of acoustic neuroma with high resolution fast spin echo T2-weighted sequence. Eur J Radiol 1997;24(1):61-65.
4. Zealley IA, Cooper RC, Clifford KM, et al. MRI screening for acoustic neuroma: a comparison of fast spin echo and contrast enhanced imaging in 1233 patients. Br J Radiol 2000;73(867):242-247.
5. Stuckey SL, Harris AJ, Mannolini SM. Detection of acoustic schwannoma: use of constructive interference in the steady state three-dimensional MR. AJNR Am J Neuroradiol 1996;17(7):1219-1225.
6. Hermans R, Van der Goten A, De Foer B, Baert AL. MRI screening for acoustic neuroma without gadolinium: value of 3DFT-CISS sequence. Neuroradiology 1997;39(8):593-598.
7. Haque S, Hossain A, Quddus MA, Jahan MU. Role of MRI in the evaluation of acoustic schwannoma and its comparison to histopathological findings. Bangladesh Med Res Counc Bull 2011;37(3):92-96.
8. Singh K, Singh MP, Thukral C, Rao K, Singh K, Singh A. Role of magnetic resonance imaging in evaluation of cerebellopontine angle schwannomas. Indian J Otolaryngol Head Neck Surg 2015;67(1):21-27.
9. Bhadelia RA, Tedesco KL, Hwang S, et al. Increased cochlear fluid-attenuated inversion recovery signal in patients with vestibular schwannoma. AJNR Am J Neuroradiol 2008;29(4):720-723.
10. Thamburaj K, Radhakrishnan VV, Thomas B, Nair S, Menon G. Intratumoral microhemorrhages on T2*-weighted gradient-echo imaging helps differentiate vestibular schwannoma from meningioma. AJNR Am J Neuroradiol 2008;29(3):552-557.
11. Held P, Fellner C, Seitz J, Graf S, Fellner F, Strutz J. The value of T2(*)-weighted MR images for the diagnosis of acoustic neuromas. Eur J Radiol 1999;30(3):237-244.
12. Held P, Fellner C, Fellner F, et al. MRI of inner ear and facial nerve pathology using 3D MP-RAGE and 3D CISS sequences. Br J Radiol 1997;70(834):558-566.
13. Ozgen B, Oguz B, Dolgun A. Diagnostic accuracy of the constructive interference in steady state sequence alone for follow-up imaging of vestibular schwannomas. AJNR Am J Neuroradiol 2009;30(5):985-991.
14. Rampp S, Scheller C, Prell J, Engelhorn T, Strauss C, Rachinger J. Magnetic resonance imaging dynamics of contrast medium uptake in vestibular schwannomas. J Neurosurg 2011;114(2):394-399.
15. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270(3):834-841.
16. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21(4):1104-1108.
17. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17(9): 2359-2362.
18. Mazzoni A, Calabrese V, Moschini L. Residual and recurrent acoustic neuroma in hearing preservation procedures: neuroradiologic and surgical findings. Skull Base Surg 1996;6(2):105-112.
19. Weissman, JL, Hirsch BE, Fukui MB, Rudy TE. The evolving MR appearance of structures in the internal auditory canal after removal of an acoustic neuroma. AJNR Am J Neuroradiol 1997;18(2):313-323.
20. Brors D, Schäfers M, Bodmer D, Draf W, Kahle G, Schick B. Postoperative magnetic resonance imaging findings after transtemporal and translabyrinthine vestibular schwannoma resection. Laryngoscope 2003;113(3):420-426.
21. Carlson ML, Van Abel KM, Driscoll CL, et al. Magnetic resonance imaging surveillance following vestibular schwannoma resection. Laryngoscope 2012;122(2):378-388.
22. Carlson ML, Van Abel KM, Schmitt WR, et al. Nodular enhancement within the internal auditory canal following retrosigmoid vestibular schwannoma resection: a unique radiological pattern. J Neurosurg 2011;115(4):835-841.
23. Kremer P, Forsting M, Hamer J, Sartor K. MR enhancement of the internal auditory canal induced by tissue implant after resection of acoustic neurinoma. AJNR Am J Neuroradiol 1998;19(1):115-118.
24. Umezu H, Seki Y. Postoperative magnetic resonance imaging after acoustic neuroma surgery: influence of packing materials in the drilled internal auditory canal on assessment of residual tumor. Neurol Med Chir (Tokyo) 1999;39(2):141-147.
25. Bennett ML, Jackson CG, Kaufmann R, Warren F. Postoperative imaging of vestibular schwannomas. Otolaryngol Head Neck Surg 2008;138(5):667-671.
26. Charabi S, Tos M, Børgesen SE, Thomsen J. Cystic acoustic neuromas. Results of translabyrinthine surgery. Arch Otolaryngol Head Neck Surg 1994;120(12):1333-1338.
27. Fundová P, Charabi S, Tos M, Thomsen J. Cystic vestibular schwannoma: surgical outcome. J Laryngol Otol 2000. 114(12): p. 935-9.
28. Tali, E.T., et al., Cystic acoustic schwannomas: MR characteristics. AJNR Am J Neuroradiol 1993;14(5):1241-1247.
29. Wallace CJ, Fong TC, Auer RN. Cystic intracranial schwannoma. Can Assoc Radiol J 1993;44(6):453-459.
30. Wandong S, Meng L, Xingang L, et al. Cystic acoustic neuroma. J Clin Neurosci 2005;12(3):253-5.
31. Yashar P, Zada G, Harris B, Giannotta SL. Extent of resection and early postoperative outcomes following removal of cystic vestibular schwannomas: surgical experience over a decade and review of the literature. Neurosurg Focus 2012;33(3):E13.
32. Zaouche S, Ionescu E, Dubreuil C, Ferber-Viart C. Pre- and intraoperative predictive factors of facial palsy in vestibular schwannoma surgery. Acta Otolaryngol 2005;125(4):363-369.
33. Benech F, Perez R, Fontanella MM, Morra B, Albera R, Ducati A. Cystic versus solid vestibular schwannomas: a series of 80 grade III-IV patients. Neurosurg Rev 2005;28(3):209-213.
34. Metwali H, Samii M, Samii A, Gerganov V. The peculiar cystic vestibular schwannoma: a single-center experience. World Neurosurg 2014;82(6):1271-1275.
35. Piccirillo E, Wiet MR, Flanagan S, et al. Cystic vestibular schwannoma: classification, management, and facial nerve outcomes. Otol Neurotol 2009;30(6):826-834.
36. Mehrotra N, Behari S, Pal L, Banerji D, Sahu RN, Jain VK. Giant vestibular schwannomas: focusing on the differences between the solid and the cystic variants. Br J Neurosurg 2008;22(4):550-556.
37. Dunn IF, Bi WL, Erkmen K, et al. Medial acoustic neuromas: clinical and surgical implications. J Neurosurg 2014;120(5):1095-1104.
38. Sinha S, Sharma BS. Cystic acoustic neuromas: surgical outcome in a series of 58 patients. J Clin Neurosci 2008;15(5):511-515.
39. Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery 1997;40(1):11-21.
40. Charabi S, Tos M, Thomsen J, Charabi B, Mantoni M. Vestibular schwannoma growth—long-term results. Acta Otolaryngol Suppl 2000;543:7-10.
41. Jones SE, Baguley DM, Moffat DA. Are facial nerve outcomes worse following surgery for cystic vestibular schwannoma? Skull Base 2007;17(5):281-284.
42. Charabi S, Mantoni M, Tos M, Thomsen J. Cystic vestibular schwannomas: neuroimaging and growth rate. J Laryngol Otol 1994;108(5):375-379.
43. Pendl G, Ganz JC, Kitz K, Eustacchio S. Acoustic neurinomas with macrocysts treated with Gamma Knife radiosurgery. Stereotact Funct Neurosurg 1996;66(suppl 1):103-111.
44. Shirato H, Sakamoto T, Takeichi N, et al. Fractionated stereotactic radiotherapy for vestibular schwannoma (VS): comparison between cystic-type and solid-type VS. Int J Radiat Oncol Biol Phys 2000;48(5):1395-1401.
45. Gerganov VM, Klinge PM, Nouri M, Stieglitz L, Samii M, Samii A. Prognostic clinical and radiological parameters for immediate facial nerve function following vestibular schwannoma surgery. Acta Neurochir (Wien) 2009;151(6):581-587.
46. Rompaey VV, Dinther Jv, Zarowski A, Offeciers E, Somers T. Fundus obliteration and facial nerve outcome in vestibular schwannoma surgery. Skull Base 2011;21(2):99-102.
47. Kobayashi M, Tsunoda A, Komatsuzaki A, Yamada I. Distance from acoustic neuroma to fundus and a postoperative facial palsy. Laryngoscope 2002;112(1):168-171.
48. Gerganov V, Nouri M, Stieglitz L, et al. Radiological factors related to pre-operative hearing levels in patients with vestibular schwannomas. J Clin Neurosci 2009;16(8):1009-1012.
49. Matthies C, Samii M, Krebs S. Management of vestibular schwannomas (acoustic neuromas): radiological features in 202 cases—their value for diagnosis and their predictive importance. Neurosurgery 1997;40(3):469-481.
50. Mohr G, Sade B, Dufour JJ, Rappaport JM. Preservation of hearing in patients undergoing microsurgery for vestibular schwannoma: degree of meatal filling. J Neurosurg 2005;102(1):1-5.
51. Jung S, Kim SH, Kim HW, et al. Prediction of facial nerve displacement in extra large vestibular schwannoma. Acta Neurochir (Wien) 1998;140(11):1143-1145.
52. Schmalbrock P, Chakeres DW, Monroe JW, et al. Assessment of internal auditory canal tumors: a comparison of contrast-enhanced T1-weighted and steady-state T2-weighted gradient-echo MR imaging. AJNR Am J Neuroradiol 1999;20(7):1207-1213.
53. Sartoretti-Schefer S, Kollias S, Valavanis A. Spatial relationship between vestibular schwannoma and facial nerve on three-dimensional T2-weighted fast spin-echo MR images. AJNR Am J Neuroradiol 2000;21(5):810-816.
54. Nakai T, Yamamoto H, Tanaka K, et al. Preoperative detection of the facial nerve by high-field magnetic resonance imaging in patients with vestibular schwannoma. Neuroradiology 2013;55(5):615-620.
55. Liang C, Zhang B, Wu L, et al. The superiority of 3D-CISS sequence in displaying the cisternal segment of facial, vestibulocochlear nerves and their abnormal changes. Eur J Radiol 2010;74(3):437-440.
56. Casselman JW, Kuhweide R, Deimling M, Ampe W, Dehaene I, Meeus L. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol 1993;14(1):47-57.
57. Shigematsu Y, Korogi Y, Hirai T, et al. Contrast-enhanced CISS MRI of vestibular schwannomas: phantom and clinical studies. J Comput Assist Tomogr 1999;23(2):224-231.
58. Chen DQ, Quan J, Guha A, et al. Three-dimensional in vivo modeling of vestibular schwannomas and surrounding cranial nerves with diffusion imaging tractography. Neurosurgery 2011;68(4):1077-1083.
59. Choi KS, Kim MS, Kwon HG, Jang SH, Kim OL. Preoperative identification of facial nerve in vestibular schwannomas surgery using diffusion tensor tractography. J Korean Neurosurg Soc 2014;56(1):11-15.
60. Taoka T, Hirabayashi H, Nakagawa H, et al. Displacement of the facial nerve course by vestibular schwannoma: preoperative visualization using diffusion tensor tractography. J Magn Reson Imaging 2006;24(5):1005-1010.
61. Gerganov VM, Giordano M, Samii M, Samii A. Diffusion tensor imaging-based fiber tracking for prediction of the position of the facial nerve in relation to large vestibular schwannomas. J Neurosurg 2011;115(6):1087-1093.
62. Kocaoglu M, Bulakbasi N, Ucoz T, et al. Comparison of contrast-enhanced T1-weighted and 3D constructive interference in steady state images for predicting outcome after hearing-preservation surgery for vestibular schwannoma. Neuroradiology 2003;45(7):476-481.
63. Zhang Y, Chen Y, Zou Y, et al. Facial nerve preservation with preoperative identification and intraoperative monitoring in large vestibular schwannoma surgery. Acta Neurochir (Wien) 2013;155(10):1857-1862.
64. Smouha EE, Yoo M, Mohr K, Davis RP. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope 2005;115(3):450-454.
65. Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg 2005;103(1):59-63.
66. Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol 2006;27(4):547-552.
67. Flint D, Fagan P, Panarese A. Conservative management of sporadic unilateral acoustic neuromas. J Laryngol Otol 2005;119(6):424-428.
68. Hoistad DL, Melnik G, Mamikoglu B, et al. Update on conservative management of acoustic neuroma. Otol Neurotol 2001;22(5):682-685.
69. Bozorg Grayeli A, Kalamarides M, Ferrary E, et al. Conservative management versus surgery for small vestibular schwannomas. Acta Otolaryngol 2005;125(10):1063-1068.
70. Moffat DA, Kasbekar A, Axon PR, Lloyd SK. Growth characteristics of vestibular schwannomas. Otol Neurotol 2012;33(6):1053-1058.
71. Martin TP, Senthil L, Chavda SV, Walsh R, Irving RM. A protocol for the conservative management of vestibular schwannomas. Otol Neurotol 2009;30(3):381-385.
72. Suryanarayanan R, Ramsden RT, Saeed SR, et al. Vestibular schwannoma: role of conservative management. J Laryngol Otol 2010;124(3):251-257.
73. Fucci MJ, Buchman CA, Brackmann DE, Berliner KI. Acoustic tumor growth: implications for treatment choices. Am J Otol 1999;20(4):495-499.
74. Fayad JN, Semaan MT, Lin J, Berliner KI, Brackmann DE. Conservative management of vestibular schwannoma: expectations based on the length of the observation period. Otol Neurotol 2014;35(7):1258-1265.
75. Solares CA, Panizza B. Vestibular schwannoma: an understanding of growth should influence management decisions. Otol Neurotol 2008;29(6):829-834.
76. Bakkouri WE, Kania RE, Guichard JP, et al. Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. J Neurosurg 2009;110(4):662-669.
77. Tschudi DC, Linder TE, Fisch U. Conservative management of unilateral acoustic neuromas. Am J Otol 2000;21(5):722-728.
78. Ferri GG, Modugno GC, Pirodda A, et al. Conservative management of vestibular schwannomas: an effective strategy. Laryngoscope 2008;118(6):951-957.
79. Artz JC, Timmer FC, Mulder JJ, Cremers CW, Graamans K. Predictors of future growth of sporadic vestibular schwannomas obtained by history and radiologic assessment of the tumor. Eur Arch Otorhinolaryngol 2009;266(5):641-646.
80. Varughese JK, Breivik CN, Wentzel-Larsen T, Lund-Johansen M. Growth of untreated vestibular schwannoma: a prospective study. J Neurosurg 2012;116(4):706-712.
81. Tysome JR, Macfarlane R, Durie-Gair J, et al. Surgical management of vestibular schwannomas and hearing rehabilitation in neurofibromatosis type 2. Otol Neurotol 2012;33(3):466-472.
82. Schmerber S, Palombi O, Boubagra K, et al. Long-term control of vestibular schwannoma after a translabyrinthine complete removal. Neurosurgery 2005;57(4):693-698.
83. Arlt F, Trantakis C, Seifert V, et al. Recurrence rate, time to progression and facial nerve function in microsurgery of vestibular schwannoma. Neurol Res 2011;33(10):1032-1037.
84. Fukuda M, Oishi M, Hiraishi T, Natsumeda M, Fujii Y. Clinicopathological factors related to regrowth of vestibular schwannoma after incomplete resection. J Neurosurg 2011;114(5):1224-1231.
85. Godefroy WP, van der Mey AG, de Bruine FT, Hoekstra ER, Malessy MJ. Surgery for large vestibular schwannoma: residual tumor and outcome. Otol Neurotol 2009;30(5):629-634.
86. Tang S, Griffin AS, Waksal JA, et al. Surveillance after resection of vestibular schwannoma: measurement techniques and predictors of growth. Otol Neurotol 2014;35(7):1271-1276.
87. Lemée JM, Delahaye C, Laccourreye L, Mercier P, Fournier HD. Post-surgical vestibular schwannoma remnant tumors: what to do? Neurochirurgie 2014;60(5):205-215.
88. Bloch DC, Oghalai JS, Jackler RK, Osofsky M, Pitts LH. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg 2004;130(1):104-112.
89. Kameyama, S., et al., Long-term follow-up of the residual intracanalicular tumours after subtotal removal of acoustic neurinomas. Acta Neurochir (Wien), 1996. 138(2): p. 206-9.
90. Lye RH, Pace-Balzan A, Ramsden RT, Gillespie JE, Dutton JM. The fate of tumour rests following removal of acoustic neuromas: an MRI Gd-DTPA study. Br J Neurosurg 1992;6(3):195-201.
91. Kemink JL, Langman AW, Niparko JK, Graham MD. Operative management of acoustic neuromas: the priority of neurologic function over complete resection. Otolaryngol Head Neck Surg 1991;104(1):96-99.
92. Peyre M, Goutagny S, Imbead S, et al. Increased growth rate of vestibular schwannoma after resection of contralateral tumor in neurofibromatosis type 2. Neuro Oncol 2011;13(10):1125-1132.
93. Lee WJ, Isaacson JE. Postoperative imaging and follow-up of vestibular schwannomas. Otol Neurotol 2005;26(1):102-104.
94. Harris GJ, Plotkin SR, Maccollin M, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery 2008;62(6):1314-1319.
95. Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology 2013;81(21 suppl 1):S33-S40.
96. Slattery WH, Lev MH, Fisher LM, Connell SS, et al. MRI evaluation of neurofibromatosis 2 patients: a standardized approach for accuracy in interpretation. Otol Neurotol 2005;26(4):733-740.
97. Slattery 3rd WH, Fisher LM, Igbal Z, Oppenheimer M. Vestibular schwannoma growth rates in neurofibromatosis type 2 natural history consortium subjects. Otol Neurotol 2004;25(5):811-817.
98. Dirks MS, Butman JA, Kim HJ, et al. Long-term natural history of neurofibromatosis type 2-associated intracranial tumors. J Neurosurg 2012;117(1):109-117.
99. Ito E, Saito K, Yatsuya H, Nagatani T, Otsuka G. Factors predicting growth of vestibular schwannoma in neurofibromatosis type 2. Neurosurg Rev 2009;32(4):425-433.
100. Choi JW, Lee JY, Phi JH, et al. Clinical course of vestibular schwannoma in pediatric neurofibromatosis type 2. J Neurosurg Pediatr 2014;13(6):650-657.
101. Goutagny S, Bah AB, Parfait B, Sterkers O, Kalamarides M. Neurofibromatosis type 2 in the elderly population: clinical and molecular features. Am J Med Genet A 2013;161A(4):667-670.
102. Fisher LM, Doherty JK, Lev MH, Slattery WH. Concordance of bilateral vestibular schwannoma growth and hearing changes in neurofibromatosis 2: neurofibromatosis 2 natural history consortium. Otol Neurotol 2009;30(6):835-841.
103. Kondziolka D, Subach BR, Lunsford LD, Bissonette DJ, Flickinger JC. Outcomes after gamma knife radiosurgery in solitary acoustic tumors and neurofibromatosis type 2. Neurosurg Focus 1998;5(3):e2.
104. Subach BR, Kondziolka D, Lunsford LD, et al. Stereotactic radiosurgery in the management of acoustic neuromas associated with neurofibromatosis type 2. J Neurosurg 1999;90(5):815-822.
105. Mallory GW, Pollock BE, Foote RL, et al. Stereotactic radiosurgery for neurofibromatosis 2-associated vestibular schwannomas: toward dose optimization for tumor control and functional outcomes. Neurosurgery 2014;74(3):292-300.
© Congress of Neurological Surgeons
Source: Neurosurgery, February 2018